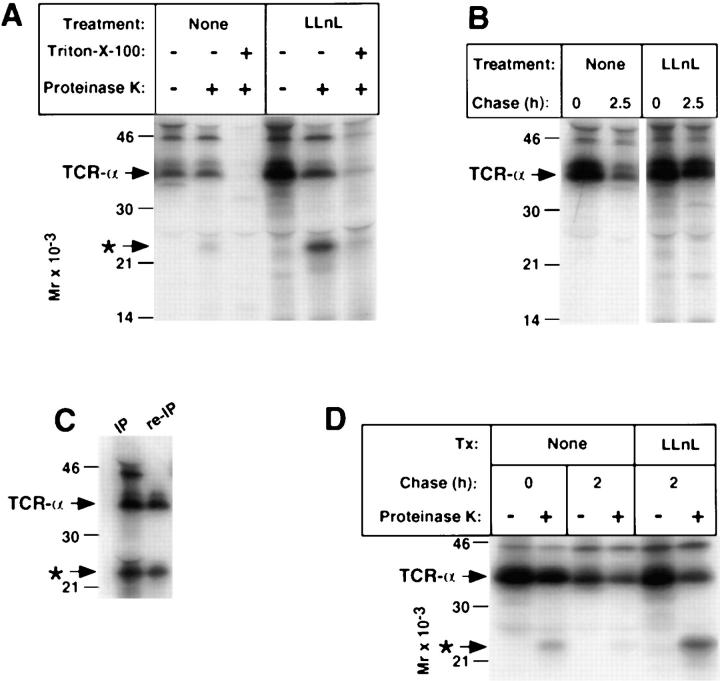
Figure 8.
Protection of TCR-α in BW5147 cells. (A) Metabolically labeled cells were subject to a 2.5-h chase followed by breakage by mechanical shearing and treatment with proteinase K as described in Fig. 4. Triton X-100 lanes indicate samples that were detergent solubilized before exposure to proteinase K. Immunoprecipitates from aliquots of cells that were not subject to breakage by mechanical shearing but instead directly lysed in Triton X-100 from the pulse and chase time points are shown in B. (C) To insure that the ∼23-kD band indicated by the asterisk represented a fragment of TCR-α and not an associated protein, a proteinase K-treated sample was immunoprecipitated with anti–TCR-α and half of the sample was denatured as described in Fig. 2, followed by reimmunoprecipitation with additional anti–TCR-α. Note that the proteinase K-dependent band (asterisk) persisted, whereas other species above TCR-α were not reimmunoprecipitated. (D) Cells were labeled with [35S]methionine in the absence of LLnL and then a 2-h chase was carried out either in the presence or absence of LLnL. This was followed by proteinase K digestion.