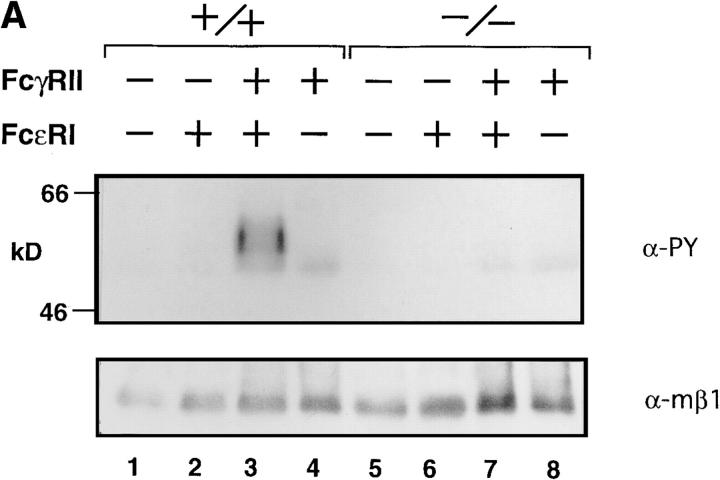
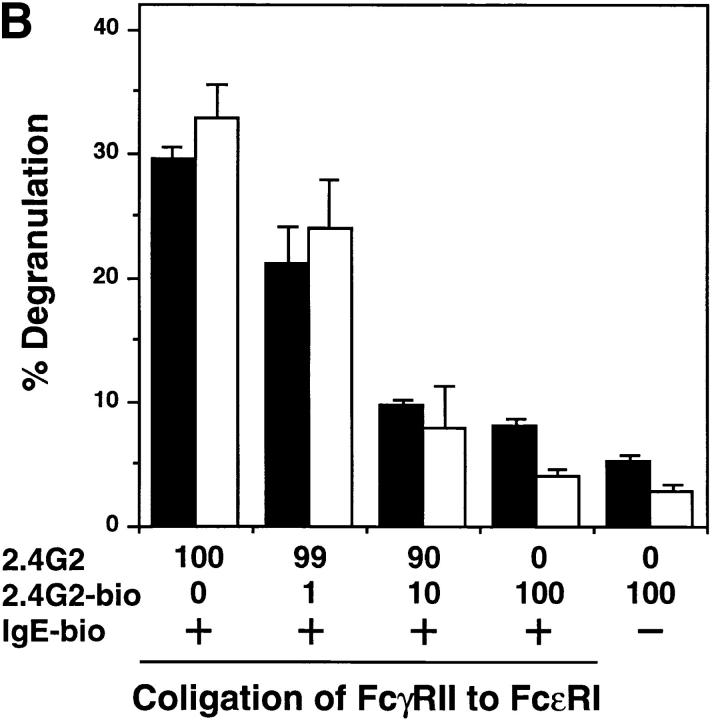
Figure 4.
(A) Tyrosine phosphorylation of FcγRIIB upon FcεRI and FcγRIIB coligation. BMMCs from wild-type (lanes 1–4), lyn −/− (lanes 5–8) mice were sensitized with anti-DNP monoclonal IgE (10 μg/ml) followed by stimulation for 2 min at 37°C with 30 ng/ml DNP-HSA (FcεRI cross-linking; lanes 2 and 6), or DNP-HSA/rabbit anti-HSA IgG immune complexes (FcγRII coligation to FcεRI; lanes 3 and 7). BMMCs were stimulated with DNP-HSA/rabbit anti-HSA IgG immune complexes without IgE sensitization (lanes 4 and 8). Anti-FcγRIIB (2.4G2) immunoprecipitates from the cell lysates were probed with α-PY (top) or anti-FcγRIIB antibody (α-mβ1) (bottom) by immunoblotting. (B) Inhibitory effect of FcγRIIB coligation to FcεRI in lyn −/− BMMCs. The mast cell was sensitized with biotinylated mouse IgE, followed by cross-linking with streptavidin (no FcγRII coligation). FcγRIIB was coligated to FcεRI by adding biotinylated anti-FcγRII monoclonal antibody (biotin-2.4G2) at the sensitization step (100% FcγRII coligation). A mixture of biotinylated/nonbiotinylated 2.4G2 (1/9 for 10% or 1/99 for 1% coligation) was used to vary the extent of FcγRII coligation. The degree of degranulation was determined by measuring the release of β-hexosaminidase as described (12). Closed columns, the results of wild-type mice; open columns, the results of lyn −/− mice. Standard errors of triplicate samples are indicated on each column.