Figure 6.
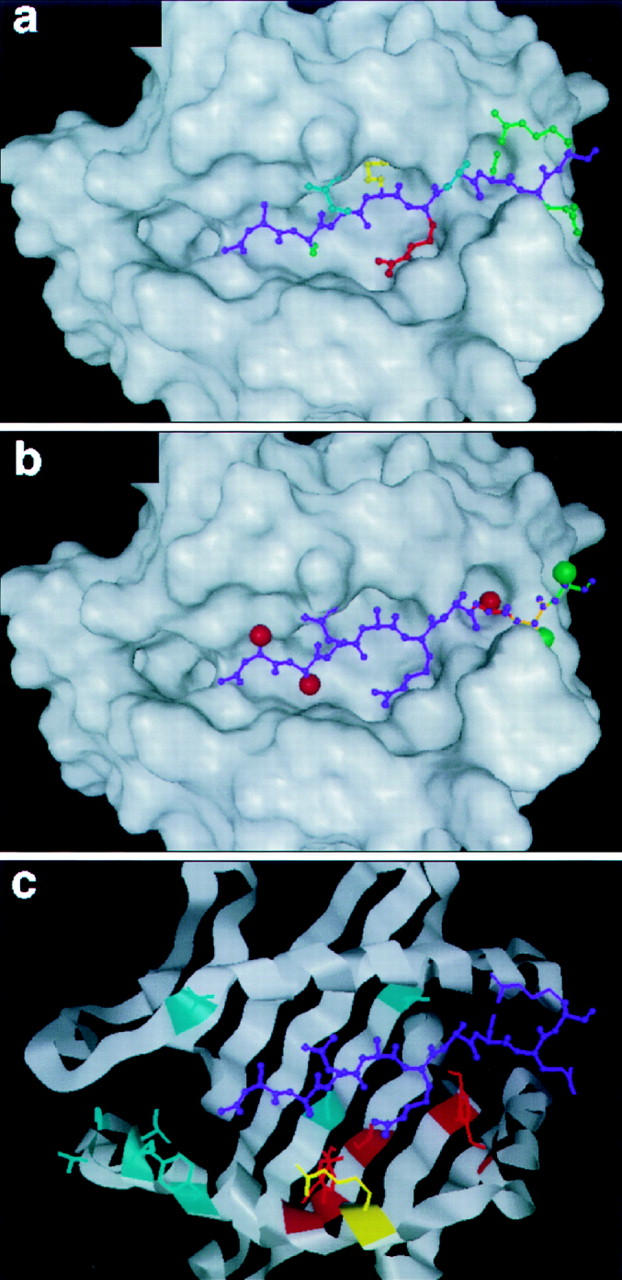
Comparison of the MBP Ac1-9–I-Au model with published data. MBP sidechains pointing towards the T cell receptor (blue), intermediate between T cell receptor and the MHC (red), buried in an MHC pocket (yellow), or interacting relatively little with either (green). These features closely match the pattern of MBP interactions with TCR–MHC as mapped by alanine-scanning substitutions (see text). Structural analysis of d-ala substitutions and COOH-terminal deletions of MBP. MBP residues 1, 2, and 7 can not accept d-ala sterically (red spheres), while residue 8 and beyond have little constraint against d-ala (green spheres). Residue 9 and beyond (green backbone) have no mainchain hydrogen bonds to the MHC, whereas deletion of residues 8 (orange) and 7 (red) would remove one and two hydrogen bonds, respectively. This is in good agreement with experimental assay of the effects of d-ala substitutions or COOH-terminal deletions on MBP–I-Au binding (see text). Amino acid substitution sites in I-Au that have no effect on MBP binding (blue), cause diminished binding (red), or improved binding (yellow). This fits the model's predicted pattern of MBP–MHC interactions well (see text).
