Abstract
To elucidate the role of A1, a new member of the Bcl-2 family of apoptosis regulators active in hematopoietic cell apoptosis, we established mice lacking A1-a, a subtype of the A1 gene in mice (A1-a−/− mice). Spontaneous apoptosis of peripheral blood neutrophils of A1-a−/− mice was enhanced compared with that of either wild-type mice or heterozygous mutants (A1-a+/− mice). Neutrophil apoptosis inhibition induced by lipopolysaccharide treatment in vitro or transendothelial migration in vivo observed in wild-type mice was abolished in both A1-a−/− and A1-a+/− animals. On the other hand, the extent of tumor necrosis factor α–induced acceleration of neutrophil apoptosis did not differ among A1-a−/−, A1-a+/−, and wild-type mice. The descending order of A1 mRNA expression was wild-type, A1-a+/−, and A1-a−/−. Taken together, these results suggest that A1 is involved in inhibition of certain types of neutrophil apoptosis.
Keywords: neutrophil, apoptosis, A1, bcl-2–related gene, gene disruption
Control of neutrophil function seems important for retaining an effective level of bacterial killing and other physiological properties while avoiding damage to normal tissues. The short life of neutrophils may ensure fulfillment of the latter requirement, since an early death after accomplishing their physiological task may prevent possible damage to normal tissues, which might occur with a prolonged survival of these cells. However, there are situations that require neutrophils to function actively for an extended period, such as during bacterial infection. In fact, these cells have been found to survive for longer periods in various situations. We have demonstrated previously that apoptosis of peritoneal exudate neutrophils (PEN)1 and peripheral blood neutrophils (PBN) obtained from rats intraperitoneally injected with inflammatory agents is severely inhibited (1). On the other hand, certain agents accelerate apoptosis of these cells. TNF-α enhances apoptosis of human (2) and rat (1) neutrophils, and PMA induces prompt neutrophil death, although in a different manner from that of typical apoptosis or necrosis (3). However, the mechanisms responsible for the regulation of neutrophil apoptosis remain largely unknown.
Several genes have been implicated in the regulation of cell death, including those of the bcl-2 family (4–9), Fas/ Apo1 (10, 11), c-myc (12), p53 (13), and nur77 (14). Bcl-2, the bcl-2 product, blocks or delays cell death after administration of death-inducing agents or growth factor withdrawal (4). Analysis of bcl-2–deficient mice provided evidence that bcl-2 is required for ensuring the full life span of mature lymphocytes in vivo (15–17). A newly recognized member of the bcl-2 family, A1, was originally isolated from a cDNA library prepared from GM-CSF–treated mouse bone marrow cultures. Murine A1 is expressed in the thymus, spleen, and bone marrow, and specifically in the hematopoietic cell lineages including Th cells, macrophages, and neutrophils (9). Recently, a role for A1 in protection against apoptosis was reported (18–20). It has also been demonstrated that A1 is the only known Bcl-2 family member to be induced by the inflammatory cytokines TNF-α and IL-1β (21).
It has been shown that Bcl-2 and Bcl-xL can inhibit most apoptosis. Both of these proteins are absent in mature neutrophils although Bcl-2 is expressed in early myeloid cells of the bone marrow (22). Expression of a newly recognized member of the Bcl-2 family, A1, alone of all the known proteins that inhibit neutrophil apoptosis, suggests that A1 plays a major role in the prevention of this apoptosis. We have reported previously that in the murine genome A1 consists of at least four genes, A1-a, -b, -c, and -d (23), all of which have a high degree of homology with each other at the nucleotide and amino acid sequence levels. In this study, we used gene targeting to establish mice lacking A1-a, one of the A1 subtypes (A1-a−/− mice), in order to investigate the possible role of A1-a in the regulation of neutrophil apoptosis. We describe here acceleration of neutrophil apoptosis in A1-a−/− mice and discuss its possible mechanisms.
Materials and Methods
Establishment of the A1-a− /− Mouse.
Genomic DNA corresponding to the A1-a locus was isolated from a library of 129Sv mouse DNA (Stratagene Inc., La Jolla, CA). The XbaI-HindIII fragment (4 kb), containing exon 1 of the A1-a coding region (1– 140 amino acids), was deleted and replaced with a PGK-neo-polyadenylate [poly(A)] cassette. The targeting vector, pA1-a-KO-neo, contains a 1.1 kb of homology 5′, 6.0 kb 3′ of the drug-resistant gene, and a PGK-tk-poly(A) cassette. The linearized pA1-a-KO-neo was transfected into E14 embryonic stem (ES) cells. For screening of A1-a-KO targeted clones by PCR, an A1-a flanking primer (5′-CATCATAGTTTGTCATTCAGGAAG-3′) and a PGK-poly(A)-specific primer (5′-GGGTGGGGTGGGATTAGATAAATG-3′) were used. PCR-positive clones were analyzed by Southern blot hybridization to confirm that there had been homologous recombination. Mutated ES cells were microinjected into C57BL/6 blastocysts that were then implanted into uteri of pseudopregnant ICR mice to generate chimeric offspring. Male chimeric mice were mated with C57BL/6 females, and A1-a+/− mice were intercrossed to obtain A1-a−/− mice. Maintenance, transfection, and selection were carried out as described previously (24).
Animals.
A1-a−/− mice and A1-a+/− mice were maintained in the animal center of our medical school in an environment kept free of pathogenic bacteria. The genotypes of newborn mice were examined by PCR at 4–8 wk of age. PCR was performed using genomic DNA obtained from a tail biopsy specimen. Two oligonucleotide primers, 5′-ATGGCTGAGTCTGAGCTCATG-3′ and 5′-CCAACCTCCATTCCGCCGTATC-3′, were used for endogenous detection, and two other primers, 5′-CATCATAGTTTGTCATTCAGGAAG-3′ and 5′-GGGTGGGGTGGGATTAGATAAATG-3′, were used for detecting knockouts. C57BL/6 and 129Sv mice were used as controls. The mice used for experiments had body weights of 25–35 g.
Preparation of PBN.
We separated PBN from blood using a partially modified method of Tsuchida et al. (1). Heparinized peripheral blood was obtained by cardiac puncture. Blood from three to seven mice of the same group was usually pooled together to obtain a sufficient number of PBN for the apoptosis assay. RBCs were sedimented by the addition of an equal volume of 3% (wt/vol) dextran T-500 (25) (Pharmacia Biotech AB, Uppsala, Sweden) in PBS. The mixture of blood and dextran was allowed to stand in a 15-ml conical tube (Falcon FAL2096; Becton Dickinson Labware, Lincoln Park, NJ) at room temperature for 12–15 min to permit the erythrocytes to sediment. The leukocyte-rich supernatant (buffy coat) was transferred to another conical tube which was then filled with Eagle's MEM (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) and centrifuged at 650 g for 5 min at 4°C. The leukocyte-rich erythrocyte layer at the bottom was suspended in 5 ml of MEM and centrifuged on 5 ml of Ficoll-Paque (specific gravity, 1.077; Pharmacia Biotech AB) at 800 g for 10 min at 4°C. The erythrocytes in the pellet were lysed by hypotonic shock. Neutrophils were washed with MEM, resuspended in RPMI 1640 medium (GIBCO BRL, Gaithersburg, MD) supplemented with 10% FCS (Whittaker M.A. Bioproducts, Inc., Walkersville, MD), and used for experiments. The purity obtained was ∼85–95%.
Preparation of PEN.
Mice were injected intraperitoneally with 4–5 ml of 3% proteose peptone (Difco Laboratories Inc., Detroit, MI). 12 h later each mouse received the same injection again. 3 h after the second injection mice were killed and PEN were harvested twice by peritoneal lavage with 4 ml of cold heparinized MEM. The cell suspension was washed by centrifugation at 650 g for 5 min at 4°C and the pellet was resuspended in 5 ml MEM and centrifuged on 5 ml of Ficoll-Paque at 800 g for 10 min at 4°C. The neutrophils were washed with MEM and resuspended in RPMI 1640 supplemented with 10% FCS and then used for experiments. The approximate purity obtained was >95%.
Neutrophil Culture.
Aliquots (0.4 ml) of neutrophil suspensions (0.5 × 106/ml) were seeded into 8-well culture slides (Falcon FAL4118; Becton Dickinson Labware) and incubated at 37°C for varying periods of time in a 5% CO2 atmosphere. Neutrophils were cultured in the presence or absence of recombinant human TNF-α (gift from Dainippon Pharmaceutical Co., Ltd., Tokyo, Japan) or LPS (Difco Laboratories Inc.). The concentration of endotoxin in the FCS used was <100 pg/ml.
Examination of Neutrophil Apoptosis.
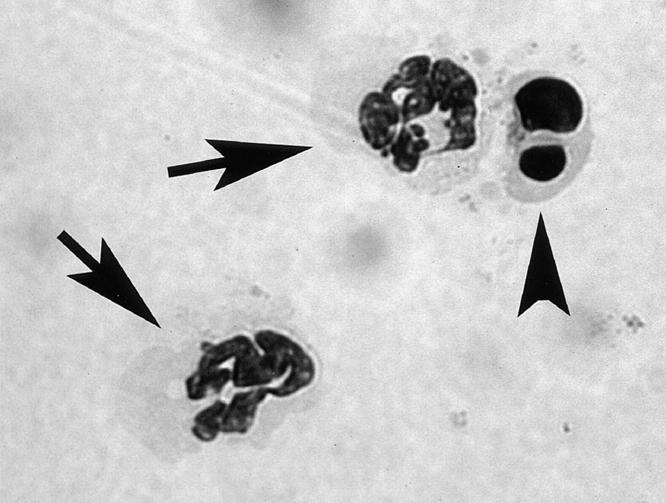
Neutrophils incubated under specific conditions were stained with May-Giemsa and apoptotic cells were counted under light microscopy (×1,000). Representative microscopic images of normal and apoptotic neutrophils are shown in Fig. 1. Approximately 300–1200 cells were scanned per specimen.
Figure 1.
Representative microscopic images of normal and apoptotic neutrophils. Aliquots (0.4 ml) of PBN suspensions of A1-a−/− mice (0.5 × 106/ml) were incubated at 37°C for 12 h in RPMI 1640 medium. Arrows indicate normal neutrophils and arrowhead indicates an apoptotic neutrophil containing condensed and fragmented nuclei (original magnification, ×1,000, May-Giemsa stain).
Detection of A1 mRNA by RT-PCR and Its Quantification by Competitive RT-PCR.
To detect A1 mRNA, the total RNA (1 μg) isolated from neutrophils was reverse transcribed with AMV reverse transcriptase (TaKaRa, Ohtsu, Japan) using an oligo dT primer. First-strand cDNA synthesis products were amplified in reaction volumes of 50 μl using the primers 5′-AATTCCAACAGCCTCCAGATATG-3′ and 5′-GAAACAAAATATCTGCAACTCTGG-3′, together with 0.625 U Ex-Taq polymerase (TaKaRa) in a DNA Thermal Cycler (Perkin-Elmer Corp., Norwalk, CT). PCR products were digested with 10 U of the restriction enzymes, BglII and NsiI (Boehringer Mannheim Biochemicals, Indianapolis, IN), to distinguish each subtype, and then electrophoresed on 2% agarose gels. The agarose gel image was captured by densitometer and relative quantification of each band was determined by NIH image analysis. To quantify A1 mRNA, a competitive RT-PCR was performed. The DNA competitor was amplified from λDNA with primers 5′-ATTTAGGTGACACT AT AGA A TACA A T TCCA A CAG C C TCCAGATATG - 3 ′ and 5′-GAAACAAAATATCTGCAACTCTGGAGAGTTTCTGCGGCAGTTAA-3′ using a competitive DNA construction kit (TaKaRa). 1–109 copies of the DNA competitors were prepared by a 10-fold dilution and amplified in a DNA Thermal Cycler with 1 μl of first-strand cDNA in reaction volumes of 50 μl using primers 5′-AATTCCAACAGCCTCCAGATATG-3′ and 5′-GAAACAAAATATCTGCAACTCTGG-3′ together with 0.625 U Ex-Taq polymerase. PCR products were electrophoresed on 2% agarose gels, and the strengths of target bands were compared visually with the competitor bands for each lane.
Data Analysis.
Results were expressed as means of at least five independent experiments. Statistical significance was determined by the unpaired t test.
Results
A1-a Gene Targeting.
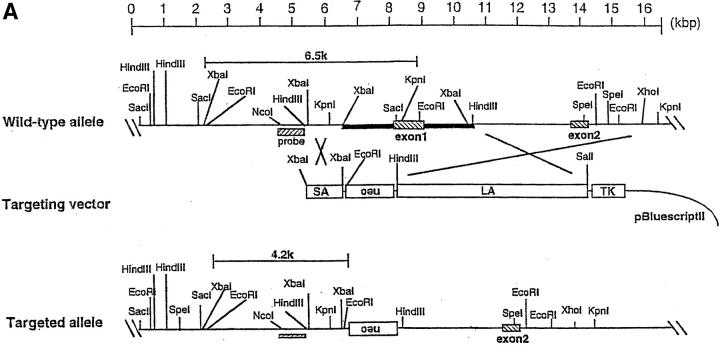
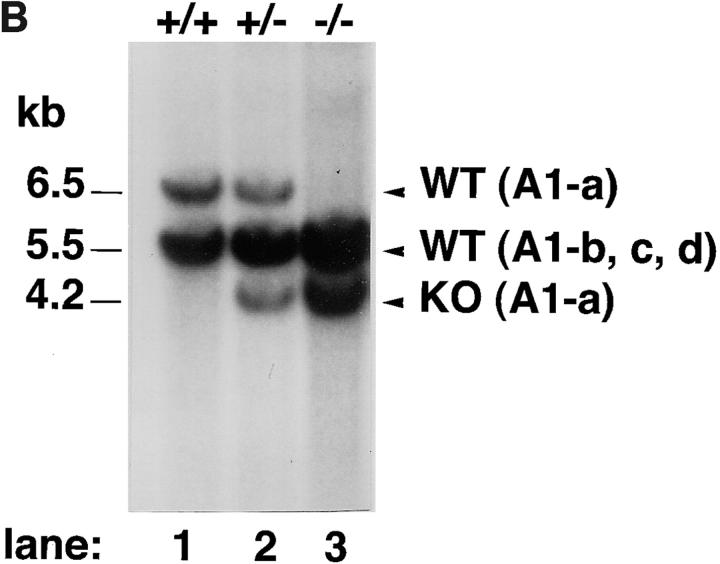
The murine A1 genomic locus is comprised of two exons spanning ∼4.5 kb. The targeting construct was designed to delete the protein coding region of exon 1 (Fig. 2 A). Electroporation of the linearized targeting vector and G418/gancyclovir selection for homologous recombinants were carried out as described in Materials and Methods. G418/gancyclovir double-resistant ES cell clones (67 clones) were screened for homologous recombination events by PCR, and two clones (3.0%) were found to have recombined homologously. All of these clones were confirmed by Southern blot analysis to contain the desired targeted allele (Fig. 2 B). The two clones were injected into C57BL/6 blastocysts, and high coat-color chimeric males that transmitted the mutant allele to the germline were obtained. Heterozygous offspring of chimeras appeared entirely normal and were fertile. To determine the relative expression level of mRNA of each A1 subtype, RNA obtained from neutrophils was amplified. The RT-PCR products were then digested with the restriction enzymes BglII and NsiI to distinguish each subtype. Expression ratios of each subtype were as follows: among the A1-a−/− mice, the A1-b/A1-d ratio was 3:2; and in A1-a+/− mice, the A1-a/A1-b/A1-d ratio was 4:7:6 (data not shown). Ratios for wild-type mice were as follows: among C57BL/6 mice, the A1-a/A1-b/A1-d ratio was 3:4:3; and in 129Sv mice, this ratio was 7:8:5 (23).
Figure 2.
Targeted disruption of the mouse A1-a gene. (A) Structure of the targeting vector, pA1-a-KO-neo, restriction map of the mouse A1-a gene, and structure of the mutated locus after homologous recombination. The coding exons are depicted by hatched boxes. The genomic fragment used as a probe for Southern blotting is shown as a hatched bar. neo was placed in a reverse orientation relative to the A1 transcription. The expected sizes of the EcoRI fragments that hybridize with the probe are indicated. (B) Southern blot analysis of genomic DNA extracted from ES cells. The DNA was digested with EcoRI and hybridized with the probe. The sizes of wild-type A1-a (WT (A1-a)), wild-types A1-b–d (WT A1-b, c, d), and disrupted (KO (A1-a)) alleles are shown; the genotypes are presented above the lanes.
Phenotype of A1-a− /− Mice.
Heterozygote matings yielded wild-type (+/+), heterozygous (+/−), and nullizygous (−/−) offspring at roughly the expected Mendelian ratio, thus indicating no significant embryonic lethality. At birth, these homozygotes were indistinguishable from their wild-type and heterozygous littermates. However, aging caused hair loss on the head and face of both A1-a−/− and A1-a+/− mice by 8–12 wk of age (Fig. 3).
Figure 3.
Physical appearance of A1-a−/− mice. Loss of hair around the face and head can be seen. Despite the loss of hair, the outward appearance of A1-a−/− mice was normal.
Despite the hair loss, the outward appearance of A1-a−/− and A1-a+/− mice was normal (Fig. 3). There was no evidence of illness in either of these groups in mice up to 12 mo of age.
Accelerated Spontaneous Apoptosis of PBN in A1-a− /− Mice.
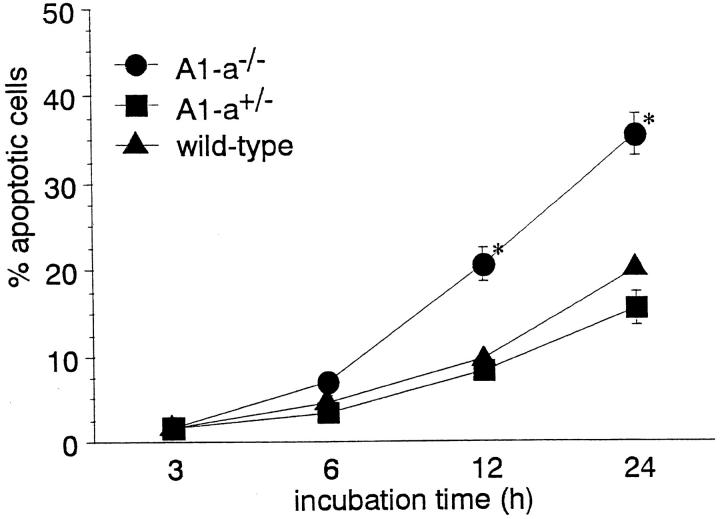
Several groups have reported that transfection of A1 into cell lines prolongs cell survival (18–20). However, there has been no report evaluating the relationship between endogenous A1 and apoptosis. To determine whether A1-a plays a role in neutrophil apoptosis, we studied the spontaneous apoptosis of PBN obtained from A1-a−/− mice. We first examined spontaneous apoptosis of PBN from control C57BL/6 and 129Sv mice. Since there was no difference in PBN apoptosis within these two strains (data not shown), we used mice of these groups as wild-type controls. Spontaneous apoptosis of PBN obtained from wild-type and A1-a+/− mice gradually increased during incubation in vitro, and there was no significant difference between these two groups. On the other hand, spontaneous apoptosis of PBN from A1-a−/− mice was significantly greater than that of wild-type or A1-a+/− mice at 12 and 24 h of incubation (Fig. 4).
Figure 4.
Spontaneous apoptosis of PBN. Aliquots (0.4 ml) of PBN (0.5 × 106/ml) were incubated for a period of up to 24 h in the absence of apoptosis-inducing agents. The numbers of apoptotic neutrophils were determined by light microscopic observation, as described in Materials and Methods. The results are expressed as means and SEs of five experiments. The unpaired t test was used to identify significant differences (*P < 0.01) between the genotypes.
Absence of Apoptosis Inhibition of PEN and LPS-treated PBN in A1-a− /− and A1-a+ /− Mice.
We and others have demonstrated that neutrophil apoptosis is modulated according to the conditions under which the cell is placed (1, 26–30). To determine whether regulation of apoptosis in similar situations can occur in A1-a−/− mice, we examined both the level of apoptosis of PEN, which was found to be lower than that of PBN in normal rats (1), and the level of apoptosis of PBN treated with LPS, which has been shown to be significantly inhibited in human preparations (26, 27). Table 1 shows that, in wild-type mice, apoptosis of neutrophils under the two sets of conditions mentioned above (PEN and LPS treatment) was significantly lower than that of untreated PBN, as already reported (1, 26, 27). On the other hand, in A1-a−/− and A1-a+/− mice, apoptosis of PEN and LPS-treated PBN was not inhibited compared with that of untreated PBN. These results indirectly suggest that A1-a may be at least partially involved in neutrophil apoptosis suppression induced by LPS treatment in vitro and transendothelial migration in vivo. Furthermore, when apoptosis of PEN and LPS-treated PBN were compared in A1-a−/−, A1-a+/−, and wild-type mice, the descending order of apoptosis was A1-a−/−, A1-a+/−, and wild-type, with significant differences observed among these groups at 12 h of incubation (Table 1).
Table 1.
Absence of Apoptosis Inhibition of PEN and LPS-treated PBN in Both A1-a− /− and A1-a+ /− Mice
| Genotype | Source of neutrophils | Treatment | % Apoptotic cells ± SE | P value using the unpaired t test (12-h incubation) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Incubation time | ||||||||||||
| 6 h | 12 h | vs. A1-a+/− | vs. Wild-type | |||||||||
| A1-a−/− | PBN | NT* | 7.1 ± 0.8 | 20.6 ± 1.9 | <0.001 | <0.001 | ||||||
| A1-a−/− | PBN | LPS‡ | 4.9 ± 0.7 | 19.0 ± 1.1 | <0.001 | <0.001 | ||||||
| A1-a−/− | PEN | NT | 7.8 ± 1.2 | 18.6 ± 1.3 | <0.001 | <0.001 | ||||||
| A1-a+/− | PBN | NT | 3.3 ± 0.5 | 8.3 ± 1.2 | 0.22 | |||||||
| A1-a+/− | PBN | LPS | 3.7 ± 0.5 | 9.2 ± 1.0 | <0.05 | |||||||
| A1-a+/− | PEN | NT | 5.1 ± 1.8 | 11.0 ± 2.2 | <0.05 | |||||||
| Wild-type | PBN | NT | 5.0 ± 0.4 | 10.4 ± 0.8 | ||||||||
| Wild-type | PBN | LPS | 2.4 ± 1.0§ | 5.2 ± 1.4§ | ||||||||
| Wild-type | PEN | NT | 1.4 ± 0.2§ | 3.8 ± 0.4§ | ||||||||
Neutrophils from the indicated source were incubated for 6 or 12 h. Experiments were conducted five times.
NT, not treated.
Neutrophils were incubated in the presence of LPS (10 μg/ml).
P < 0.01 compared with PBN NT* by the unpaired t test.
Relative Expression of A1 mRNA in Neutrophils of A1-a− /−, A1-a+ /−, and Wild-type Mice.
The above results show that neutrophil apoptosis is upregulated in both A1-a−/− and A1-a+/− mice. To begin to clarify the mechanisms involved in this phenomenon, we examined the relative expression of A1 mRNA in neutrophils obtained from mice of different genotypes. By competitive RT-PCR, the amount of A1 mRNA in neutrophils was most prominent in wild-type mice, second in A1-a+/− mice, and smallest in A1-a−/− mice (Table 2), suggesting that the level of neutrophil apoptosis in certain situations may be reflected by the level of A1 mRNA expression in these cells.
Table 2.
Relative Expression of A1 mRNA in Neutrophils Obtained from Mice with Different Genotypes
Relative expression was determined by competitive RT-PCR.
C57BL/6 A1 mRNA was weakly amplified compared with that of 129Sv. It is possible that primers designed from the sequence of 129Sv mice did not anneal properly to C57BL/6 mRNA.
No Reduction in the Number of PBN and PEN in A1-a− /− Mice.
The finding that spontaneous apoptosis of PBN and PEN was enhanced in A1-a−/− mice (Table 1) led us to speculate that the number of PBN and PEN in A1-a−/− mice might be smaller than in wild-type mice. To answer this question, we determined the number of PBN in untreated A1-a−/− , A1-a+/− , wild-type C57BL/6, and 129Sv mice. Unexpectedly, the number of PBN in these mice did not differ. Furthermore, using these four different strains of mice, we examined the number of PBN and PEN at 3, 6, 12, 24 h, as well as 3 h after a booster injection at 12 h after intraperitoneal injection of proteose peptone. The number of PBN and PEN in the four groups did not significantly differ from each other at any of the time points after the injection of the reagent (data not shown).
Lack of Change in Sensitivity to TNF-α–enhanced Neutrophil Apoptosis in A1-a− /− Mice.
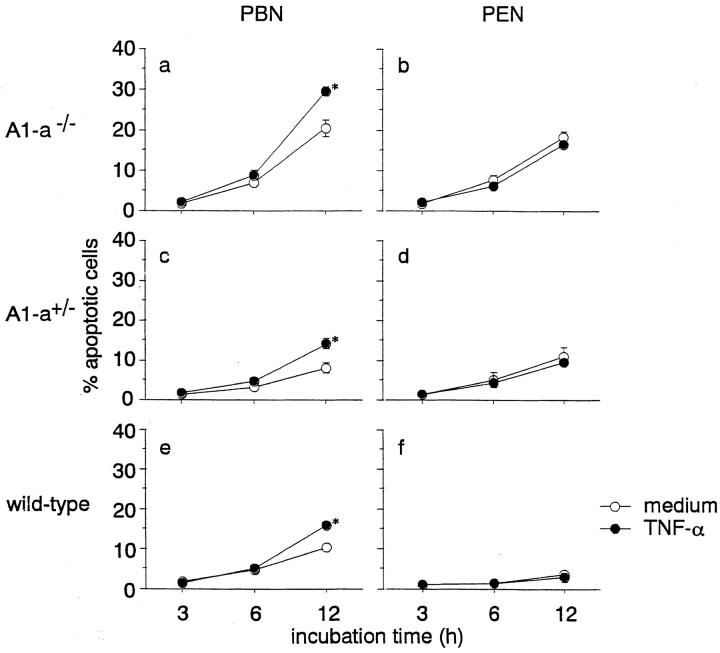
We demonstrated previously that TNF-α enhances apoptosis of human (2) and rat (1) neutrophils. To further explore possible mechanisms for the enhancement of spontaneous apoptosis of PBN in A1-a−/− mice, we examined the sensitivity of these PBN in terms of TNF-α enhancement of apoptosis. Treatment with this cytokine enhanced PBN apoptosis in all wild-type, A1-a+/−, and A1-a−/− mice at a 12 h incubation (Fig. 5). The extent of apoptosis enhancement by TNF-α was not significantly different among these three groups of mice (data not shown). On the other hand, apoptosis of PEN was not enhanced in any of the A1-a−/−, A1-a+/−, or wild-type mice by TNF-α (Fig. 5), similar to our results obtained previously with normal rats (1).
Figure 5.
Modulation of PBN and PEN apoptosis by TNF-α in A1-a−/−, A1-a+/−, and wild-type mice. Aliquots (0.4 ml, 0.5 × 106/ml) of PBN (a, c, and e) and PEN (b, d, and f) were incubated for a period of up to 12 h in the presence or absence of recombinant human TNF-α (100 U/ml). The numbers of apoptotic neutrophils were determined by light microscopic observation, as described in Materials and Methods. The results are expressed as means and SEs of five experiments. The unpaired t test was used to identify significant differences (*P < 0.01) between the groups in the presence and absence of TNF-α.
Discussion
After establishing A1-a−/− mice in this study, we were able to show that spontaneous apoptosis of PBN and PEN is enhanced in these animals. Although the mechanisms governing the short life span of neutrophils and their prompt spontaneous apoptosis remain obscure, downregulation of bcl-2 expression during the terminal phase of neutrophilic differentiation (31, 32) could largely account for the above phenomenon. It is well known that Bcl-2 inhibits apoptosis of many types of cells (4, 33) and that neutrophil apoptosis is inhibited in bcl-2 transgenic mice (34). However, other members of the Bcl-2 family are also involved in inhibiting apoptosis of various cells in certain situations. Bcl-xL, a member of the Bcl-2 family, prevents immunosuppressant-induced mature B cell apoptosis, whereas Bcl-2 is not involved in this phenomenon (35). Furthermore, Mcl-1, another member of the Bcl-2 family, delays apoptosis induced by c-Myc overexpression in Chinese hamster ovary cells (36). In this sense, A1, a new member of the Bcl-2 family, may be a main suppressor of neutrophil apoptosis for the following reasons: (a) transfection of A1 into various types of cells inhibits apoptosis (18– 20); (b) A1 is stably induced during G-CSF–stimulated neutrophilic differentiation of a myeloid precursor cell line, but transiently induced in a macrophage-like cell line or in bone marrow–derived macrophages (9); (c) mouse neutrophils express all subtypes of A1, A1-a, A1-b, and A1-d (23); (d) although overexpression of Bcl-2 inhibits spontaneous neutrophilic differentiation of a myeloid precursor cell line, overexpression of A1 does not (18) (However, this does not preclude the possibility that A1 is active in neutrophils.); and (e) furthermore, no enhancement of T cell apoptosis was observed in A1-a−/− mice (Hatakeyama, S., unpublished results), which suggests a possible role for A1 in the restrictive lineage of hematopoietic cells.
Our present finding that A1-a−/− mice exhibit enhanced neutrophil apoptosis further strengthens the assumption that A1 is a major suppressor of this process. Furthermore, the finding that knockout of the A1-a subtype results in enhancement of neutrophil apoptosis suggests that this subtype functions as an important suppressor of apoptosis of these cells. However, inasmuch as cycloheximide treatment of neutrophils of wild-type mice induces greater apoptosis than that of A1-a−/− (Hamasaki, A., unpublished results), the other subtypes, A1-b and A1-d, may also be involved in neutrophil apoptosis inhibition.
It has been shown previously that treatment of neutrophils in vitro with cytokines such as IL-1 (26), G-CSF (26), GM-CSF (26, 28, 29), and IFN-γ (26), which are detectable in inflammatory foci, inhibits apoptosis of these cells. Furthermore, endothelial transmigration in vitro (30) and in vivo (1) both delay neutrophil apoptosis. The present finding that neutrophil apoptosis inhibition by LPS in vitro and transendothelial migration in vivo were completely abrogated in A1-a−/− mice strongly suggests that A1-a is involved in this inflammation-mediated suppression of apoptosis.
The present finding that spontaneous neutrophil apoptosis is accelerated in A1-a−/− mice (Fig. 4) suggests that A1-a is also involved in this suppression of apoptosis. The constitutive expression of A1 mRNA in neutrophils of untreated wild-type mice supports this assumption. As the mechanisms involved in spontaneous neutrophil apoptosis, autocrine death induced by Fas/FasL interaction on the neutrophil surface (37), reactive oxygen intermediates (38, 39), and caspase (40), have been suggested as playing an active role in effector mechanisms, which may not necessarily be mutually exclusive, and which may actually be interactive to some degree. An examination of candidate mechanisms and the manner in which A1 modulates apoptosis will be the focus of future studies on A1 involvement in spontaneous neutrophil apoptosis inhibition.
The finding in this study that inhibition of PEN and LPS-treated PBN apoptosis was abrogated in both A1-a−/− and A1-a+/− mice, whereas acceleration of spontaneous PBN apoptosis was observed in A1-a−/− alone (Table 1), may be explained as follows: in untreated PBN, the concentration of A1 protein in A1+/− may be sufficient to inhibit apoptosis, since mRNA expression of A1 in these mice is 10-fold higher than in A1-a−/−, but only one tenth that of wild-type 129Sv mice (Table 2). However, a greater concentration of A1 protein may be required to inhibit apoptosis of LPS-treated PBN or extravasated PEN, which may explain the lack of inhibition in A1-a−/− and A1-a+/− mice.
Our finding that TNF-α–induced acceleration of neutrophil apoptosis was not augmented in A1-a−/− is apparently inconsistent with the previous finding that transfection of the A1 gene into an endothelial cell line resulted in inhibition of TNF-α–induced apoptosis of these cells (19). This apparent discrepancy may be reconciled as follows: first, although the discoveries of TNF receptor-binding cytoplasmic proteins such as TNF receptor 1-associated death domain protein (41) and TNF receptor–associated factor (42, 43) have expanded our understanding of the mechanisms of TNF-α–induced apoptosis, the picture is far from clear. Various molecules such as IL-1β–converting enzyme–like protease (44), ceramide (45, 46), reactive oxygen intermediates (47), and nicotinamide dinucleotide (48) have been proposed as candidates for mediators of TNF-α– induced apoptosis. On the other hand, nitric oxide (49) and A20 zinc finger protein (50) are both thought to be inhibitors of this form of apoptosis. Apoptosis induction and inhibition through mechanisms involving the above molecules are dependent on cell type (44–50). Therefore, signal transductions governing TNF-α–induced apoptosis may differ in neutrophils and endothelial cells. Secondly, a difference in the manner of TNF-α–induced apoptosis of the two cell types may explain the discrepancy in the results, i.e., why addition of actinomycin D is required for TNF-α– induced apoptosis of endothelial cells (19) while TNF-α alone is sufficient to induce neutrophil apoptosis (1, 2). In addition, our preliminary results suggesting that the GM-CSF–induced inhibition of neutrophil apoptosis was not abolished in A1-a−/− mice (Hamasaki, A., unpublished results) themselves suggest that there exist systems of neutrophil apoptosis regulation other than those shown in this manuscript.
The unexpected result that the number of PBN and PEN was not significantly different in A1-a−/−, A1-a+/−, and wild-type mice may be explained as follows: (a) various systems other than A1, involved in the regulation of neutrophil apoptosis may compensate for this apoptosis in vivo; (b) positive feedback regulation of PBN number may be induced in A1-a−/− mice through cytokines such as G-CSF; and (c) a less probable possibility is that preapoptotic change in cell surface carbohydrates which induce phagocytosis by macrophages (51) may not differ between A1-a−/−, A1-a+/−, and wild-type mice and this may result in the same grade of phagocytosis of preapoptotic neutrophils in these strains of mice.
Studies are now in progress to more precisely elucidate the mechanisms of A1 involvement in the inhibition of neutrophil apoptosis and to examine whether A1 is also involved in the inhibition of apoptosis of myeloid cells other than neutrophils.
Acknowledgments
We are grateful to Dr. Yuji Takeda, Akemi Araki, and Kazue Hayashi for technical assistance.
Footnotes
This work was partially supported by a Grant-in-Aid for Scientific Research (09470069) from the Ministry of Education, Science, and Culture, Japan.
Abbreviations used in this paper: ES, embryonic stem cells; PBN, peripheral blood neutrophils; PEN, peritoneal exudate neutrophils.
References
- 1.Tsuchida H, Takeda Y, Takei H, Shinzawa H, Takahashi T, Sendo F. In vivo regulation of rat neutrophil apoptosis occurring spontaneously or induced with TNF-α or cycloheximide. J Immunol. 1995;154:2403–2412. [PubMed] [Google Scholar]
- 2.Takeda Y, Watanabe H, Yonehara S, Yamashita T, Saito S, Sendo F. Rapid acceleration of neutrophil apoptosis by tumor necrosis factor-α. Int Immunol. 1993;5:691–694. doi: 10.1093/intimm/5.6.691. [DOI] [PubMed] [Google Scholar]
- 3.Takei H, Araki A, Watanabe H, Ichinose A, Sendo F. Rapid killing of human neutrophils by the potent activator phorbol 12-myristate 13-acetate (PMA) accompanied by changes different from typical apoptosis or necrosis. J Leukoc Biol. 1996;59:229–240. doi: 10.1002/jlb.59.2.229. [DOI] [PubMed] [Google Scholar]
- 4.Nuñez G, London L, Hockenbery D, Alexander M, McKearn JP, Korsmeyer SJ. Deregulated Bcl-2 gene expression selectively prolongs survival of growth factor- deprived hemopoietic cell lines. J Immunol. 1990;144:3602–3610. [PubMed] [Google Scholar]
- 5.Boise LH, González-Garcia M, Postema CE, Ding L, Lindsten T, Turka LA, Mao X, Nuñez G, Thompson CB. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- 6.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 7.Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ. Bad, a heterodimeric partner for Bcl-xLand Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80:285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- 8.Kozopas KM, Yang T, Buchan HL, Zhou P, Craig RW. MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Proc Natl Acad Sci USA. 1993;90:3516–3520. doi: 10.1073/pnas.90.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin EY, Orlofsky A, Berger MS, Prystowsky MB. Characterization of A1, a novel hemopoietic-specific early-response gene with sequence similarity to bcl-2. . J Immunol. 1993;151:1979–1988. [PubMed] [Google Scholar]
- 10.Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima S, Sameshima M, Hase A, Seto Y, Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991;66:233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- 11.Oehm A, Behrmann I, Falk W, Pawlita M, Maier G, Klas C, Li-Weber M, Richards S, Dhein J, Trauth BC, et al. Purification and molecular cloning of the APO-1 cell surface antigen, a member of the tumor necrosis factor/nerve growth factor receptor superfamily. Sequence identity with the Fas antigen. J Biol Chem. 1992;267:10709–10715. [PubMed] [Google Scholar]
- 12.Shi Y, Glynn JM, Guilbert LJ, Cotter TG, Bissonnette RP, Green DR. Role for c-mycin activation- induced apoptotic cell death in T cell hybridomas. Science. 1992;257:212–214. doi: 10.1126/science.1378649. [DOI] [PubMed] [Google Scholar]
- 13.Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993;362:847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- 14.Woronicz JD, Calnan B, Ngo V, Winoto A. Requirement for the orphan steroid receptor Nur77in apoptosis of T-cell hybridomas. Nature. 1994;367:277–281. doi: 10.1038/367277a0. [DOI] [PubMed] [Google Scholar]
- 15.Nakayama K-i, Nakayama K, Negishi I, Kuida K, Louie MC, Kanagawa O, Nakauchi H, Loh DY. Requirement for CD8 β chain in positive selection of CD8-lineage T cells. Science. 1994;263:1131–1133. doi: 10.1126/science.8108731. [DOI] [PubMed] [Google Scholar]
- 16.Nakayama K, Nakayama K-i, Negishi I, Kuida K, Sawa H, Loh DY. Targeted disruption of Bcl-2αβ in mice: occurrence of gray hair, polycystic kidney disease, and lymphocytopenia. Proc Natl Acad Sci USA. 1994;91:3700–3704. doi: 10.1073/pnas.91.9.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ. Bcl-2–deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993;75:229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- 18.Lin EY, Orlofsky A, Wang HG, Reed JC, Prystowsky MB. A1, a Bcl-2 family member, prolongs cell survival and permits myeloid differentiation. Blood. 1996;87:983–992. [PubMed] [Google Scholar]
- 19.Karsan A, Yee E, Harlan JM. Endothelial cell death induced by tumor necrosis factor-α is inhibited by the Bcl-2 family member, A1. J Biol Chem. 1996;271:27201–27204. doi: 10.1074/jbc.271.44.27201. [DOI] [PubMed] [Google Scholar]
- 20.Carrió R, López HM, Jimeno J, Benedict MA, Merino R, Benito A, Fernández LJ, Núñez G, García PJ, Merino J. A1demonstrates restricted tissue distribution during embryonic development and functions to protect against cell death. Am J Pathol. 1996;149:2133–2142. [PMC free article] [PubMed] [Google Scholar]
- 21.Karsan A, Yee E, Kaushansky K, Harlan JM. Cloning of human Bcl-2 homologue: inflammatory cytokines induce human A1 in cultured endothelial cells. Blood. 1996;87:3089–3096. [PubMed] [Google Scholar]
- 22.Ohta K, Iwai K, Kasahara Y, Taniguchi N, Krajewski S, Reed JC, Miyawaki T. Immunoblot analysis of cellular expression of Bcl-2 family proteins, Bcl-2, Bax, Bcl-X, and Mcl-1 in human peripheral blood and lymphoid tissues. Int Immunol. 1995;7:1817–1825. doi: 10.1093/intimm/7.11.1817. [DOI] [PubMed] [Google Scholar]
- 23.Hatakeyama S, Hamasaki A, Negishi I, Loh DY, Sendo F, Nakayama K, Nakayama K-i. Multiple gene duplication and expression of mouse bcl-2related genes, A1. Int Immunol. 1998;10:631–637. doi: 10.1093/intimm/10.5.631. [DOI] [PubMed] [Google Scholar]
- 24.Nakayama K-i, Nakayama K, Negishi I, Kuida K, Shinkai Y, Louie MC, Fields LE, Lucas PJ, Stewart V, Alt FW, Loh DY. Disappearance of the lymphoid system in Bcl-2 homozygous mutant chimeric mice. Science. 1993;261:1584–1588. doi: 10.1126/science.8372353. [DOI] [PubMed] [Google Scholar]
- 25.Wahba AV, Barnes B, Lazarus GS. Labeling of peripheral blood polymorphonuclear leukocytes with indium-111: a new method for the quantitation of in-vivo accumulation of PMNLs in rabbit skin. J Invest Dermatol. 1984;82:126–131. doi: 10.1111/1523-1747.ep12259669. [DOI] [PubMed] [Google Scholar]
- 26.Colotta F, Re F, Polentarutti N, Sozzani S, Mantovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood. 1992;80:2012–2020. [PubMed] [Google Scholar]
- 27.Hachiya O, Takeda Y, Miyata H, Watanabe H, Yamashita T, Sendo F. Inhibition by bacterial lipopolysaccharide of spontaneous and TNF-α–induced human neutrophil apoptosis in vitro. Microbiol Immunol. 1995;39:715–723. doi: 10.1111/j.1348-0421.1995.tb03247.x. [DOI] [PubMed] [Google Scholar]
- 28.Brach MA, deVos S, Gruss HJ, Herrmann F. Prolongation of survival of human polymorphonuclear neutrophils by granulocyte-macrophage colony-stimulating factor is caused by inhibition of programmed cell death. Blood. 1992;80:2920–2924. [PubMed] [Google Scholar]
- 29.Lee A, Whyte MK, Haslett C. Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J Leukoc Biol. 1993;54:283–288. [PubMed] [Google Scholar]
- 30.Watson RW, Rotstein OD, Nathens AB, Parodo J, Marshall JC. Neutrophil apoptosis is modulated by endothelial transmigration and adhesion molecule engagement. J Immunol. 1997;158:945–953. [PubMed] [Google Scholar]
- 31.Naumovski L, Cleary ML. Bcl2 inhibits apoptosis associated with terminal differentiation of HL-60 myeloid leukemia cells. Blood. 1994;83:2261–2267. [PubMed] [Google Scholar]
- 32.Delia D, Aiello A, Soligo D, Fontanella E, Melani C, Pezzella F, Pierotti MA, Della G, Porta bcl-2proto-oncogene expression in normal and neoplastic human myeloid cells. Blood. 1992;79:1291–1298. [PubMed] [Google Scholar]
- 33.Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-mycto immortalize pre-B cells. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 34.Lagasse E, Weissman IL. Bcl-2 inhibits apoptosis of neutrophils but not their engulfment by macrophages. J Exp Med. 1994;179:1047–1052. doi: 10.1084/jem.179.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gottschalk AR, Boise LH, Thompson CB, Quintans J. Identification of immunosuppressant-induced apoptosis in a murine B-cell line and its prevention by bcl-x but not bcl-2. Proc Natl Acad Sci USA. 1994;91:7350–7354. doi: 10.1073/pnas.91.15.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reynolds JE, Yang T, Qian L, Jenkinson JD, Zhou P, Eastman A, Craig RW. Mcl-1, a member of the Bcl-2 family, delays apoptosis induced by c-Myc overexpression in Chinese hamster ovary cells. Cancer Res. 1994;54:6348–6352. [PubMed] [Google Scholar]
- 37.Liles WC, Kiener PA, Ledbetter JA, Aruffo A, Klebanoff SJ. Differential expression of Fas (CD95) and Fas ligand on normal human phagocytes: implications for the regulation of apoptosis in neutrophils. J Exp Med. 1996;184:429–440. doi: 10.1084/jem.184.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kasahara Y, Iwai K, Yachie A, Ohta K, Konno A, Seki H, Miyawaki T, Taniguchi N. Involvement of reactive oxygen intermediates in spontaneous and CD95 (Fas/APO-1)-mediated apoptosis of neutrophils. Blood. 1997;89:1748–1753. [PubMed] [Google Scholar]
- 39.Narayanan PK, Ragheb K, Lawler G, Robinson JP. Defects in intracellular oxidative metabolism of neutrophils undergoing apoptosis. J Leukoc Biol. 1997;61:481–488. doi: 10.1002/jlb.61.4.481. [DOI] [PubMed] [Google Scholar]
- 40.William R, Watson G, Rotstein OD, Parodo J, Bitar R, Hackam D, Marshall JC. Granulocytic differentiation of HL-60 cells results in spontaneous apoptosis mediated by increased caspase expression. FEBS (Fed Eur Biochem Soc) Lett. 1997;412:603–609. doi: 10.1016/s0014-5793(97)00779-5. [DOI] [PubMed] [Google Scholar]
- 41.Hsu H, Xiong J, Goeddel DV. The TNF receptor 1-associated protein TRADD signals cell death and NF-κB activation. Cell. 1995;81:495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 42.Rothe M, Wong SC, Henzel WJ, Goeddel DV. A novel family of putative signal transducers associated with the cytoplasmic domain of the 75 kDa tumor necrosis factor receptor. Cell. 1994;78:681–692. doi: 10.1016/0092-8674(94)90532-0. [DOI] [PubMed] [Google Scholar]
- 43.Rothe M, Sarma V, Dixit VM, Goeddel DV. TRAF2-mediated activation of NF-κB by TNF receptor 2 and CD40. Science. 1995;269:1424–1427. doi: 10.1126/science.7544915. [DOI] [PubMed] [Google Scholar]
- 44.Enari M, Hug H, Nagata S. Involvement of an ICE-like protease in Fas-mediated apoptosis. Nature. 1995;375:78–81. doi: 10.1038/375078a0. [DOI] [PubMed] [Google Scholar]
- 45.Obeid LM, Linardic CM, Karolak LA, Hannun YA. Programmed cell death induced by ceramide. Science. 1993;259:1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- 46.Jarvis WD, Kolesnick RN, Fornari FA, Traylor RS, Gewirtz DA, Grant S. Induction of apoptotic DNA damage and cell death by activation of the sphingomyelin pathway. Proc Natl Acad Sci USA. 1994;91:73–77. doi: 10.1073/pnas.91.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schulze-Osthoff K, Krammer PH, Droge W. Divergent signaling via APO-1/Fas and the TNF receptor, two homologous molecules involved in physiological cell death. EMBO (Eur Mol Biol Organ) J. 1994;13:4587–4596. doi: 10.1002/j.1460-2075.1994.tb06780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wright SC, Wei QS, Kinder DH, Larrick JW. Biochemical pathways of apoptosis: nicotinamide adenine dinucleotide–deficient cells are resistant to tumor necrosis factor or ultraviolet light activation of the 24-kD apoptotic protease and DNA fragmentation. J Exp Med. 1996;183:463–471. doi: 10.1084/jem.183.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dimmeler S, Haendeler J, Nehls M, Zeiher AM. Suppression of apoptosis by nitric oxide via inhibition of interleukin-1β-converting enzyme (ICE)-like and cysteine protease protein (CPP)-32-like proteases. J Exp Med. 1997;185:601–607. doi: 10.1084/jem.185.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jaattela M, Mouritzen H, Elling F, Bastholm L. A20 zinc finger protein inhibits TNF and IL-1 signaling. J Immunol. 1996;156:1166–1173. [PubMed] [Google Scholar]
- 51.Savill J, Dransfield I, Hogg N, Haslett C. Vitronectin receptor–mediated phagocytosis of cells undergoing apoptosis. Nature. 1990;343:170–173. doi: 10.1038/343170a0. [DOI] [PubMed] [Google Scholar]