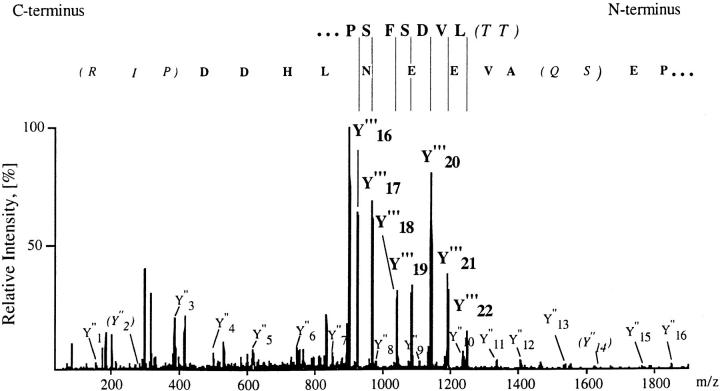
Figure 1.
(Top) Nanoelectrospray tandem mass spectrometric sequencing of a 25-residue peptide of pp29/30, showing the tandem mass spectrum acquired from the triply charged ion at m/z = 900.4. Collisional fragmentation of triply charged ions of tryptic peptides typically results in overlapping series of COOH-terminal peptide fragments (Y-ions) bearing two positive charges (Y′′′, bold) or one positive charge (Y′′). The amino acid sequence is deduced from the spectrum by considering the precise mass differences between adjacent peptide fragments in conjunction with the known amino acid molecular weights. The series of doubly charged Y′′′-ions allowed determination of the sequence stretch starting from the NH2 terminus of the peptide (top row). The sequence was then extended to the COOH terminus by following the series of singly charged Y′′-ions (bottom row), which partially overlaps with the series of doubly charged fragments. The interpretation algorithm used here relies heavily on the correct identification of the Y-ions among the other fragment ions in the spectrum. In three regions of the spectrum, the amino acid sequence was called incorrectly, because of the very low intensity of the relevant Y′′-ions (ions Y′′23, Y′′14, and Y′′2). Sequence stretches confirmed after the full-length cloning of the protein are in bold, and the regions where the correct sequence was not obtained are shown in parentheses (the correct sequence determined after the cloning is inside the parentheses in italics). In all cases, the sequence obtained from the cloned protein was consistent with the tandem mass spectrum. Altogether, two contiguous sequence stretches each of nine amino acid residues were accurately called.