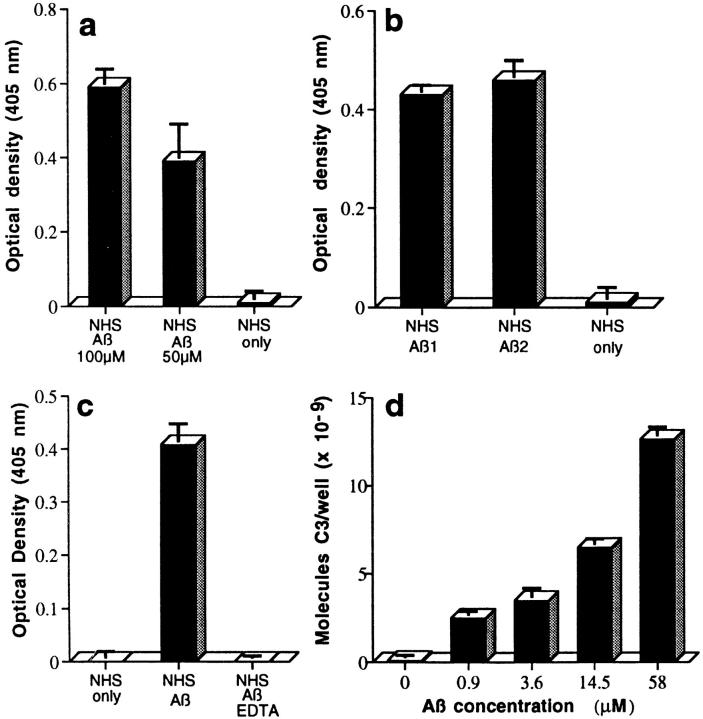
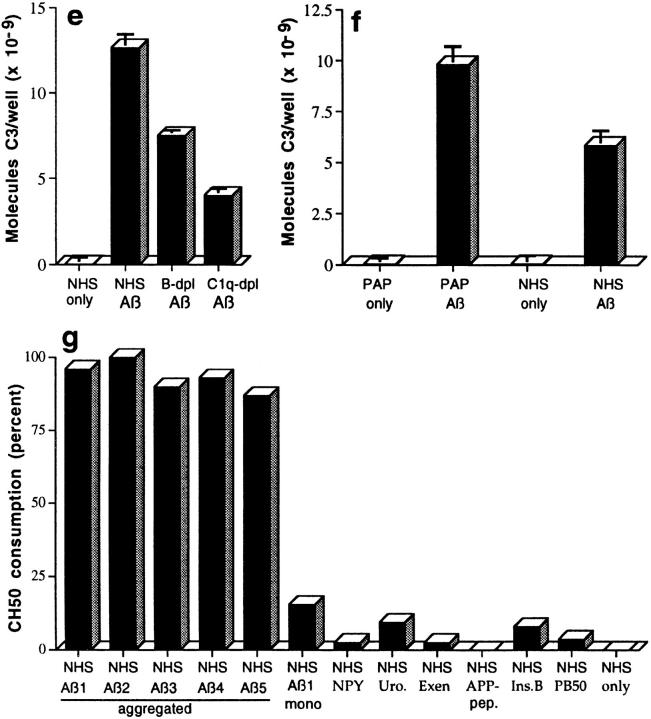
Figure 1.
ELISA demonstration of complement-mediated formation of complexes of Aβ with C3 activation fragments (a– d ). Complexes were captured, detected, and quantitated as described in Materials and Methods. NHS or purified ACP proteins only do not contain Aβ. (a) Preaggregated Aβ 1–42 (100 μM and 50 μM) was incubated in NHS, captured with mAb to C3b, and detected with rabbit Ab to Aβ. (b) Two preaggregated Aβ 1–42 preparations (Aβ 1 and Aβ 2, at 20 μM) were incubated in NHS, captured with mAb to iC3b, and detected with rabbit Ab to Aβ. (c) Preaggregated Aβ 1–42 (58 μM) was incubated in NHS, or in NHS containing 10 mM EDTA, captured with mAb to Aβ, and detected with rabbit Ab to C3. (d ) Preaggregated Aβ 1–42 was incubated in NHS at the indicated final concentrations, captured with mAb to Aβ, and detected with rabbit Ab to C3. (e) Preaggregated Aβ 1–42 (58 μM) was incubated in NHS, factor B–depleted NHS (B-dpl ), or C1q-depleted NHS (C1q-dpl ), captured with mAb to Aβ, and detected with rabbit Ab to C3. (f ) Preaggregated Aβ 1–42 (58 μM) was incubated with the six purified ACP proteins (PAP) or NHS, captured with mAb to Aβ, and detected with rabbit Ab to C3. Background levels obtained in EDTA controls containing Aβ and purified ACP proteins or NHS, in the various experiments described above, were subtracted. ( g) Specificity of complement activation. Preaggregated Aβ preparations (20 μM) and the same concentrations of monomeric Aβ (mono), insulin B chain (Ins.B), neuropeptide Y-porcine (NPY), urotensin I (Uro.), exendin 3 (Exen), amyloid precursor peptide 657–676 (APP-pep.), and adenovirus penton base 50-residue peptide (PB50) were incubated with NHS. Complement activation was assessed by the CH50 method. Correlation coefficients for the CH50 determinations ranged from 0.995 to 1.000.