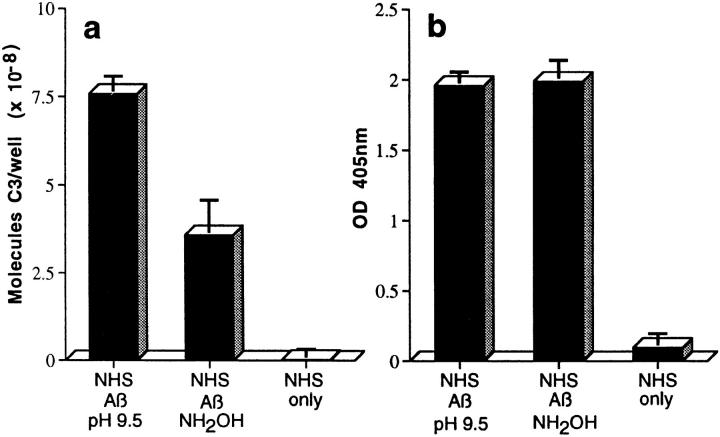
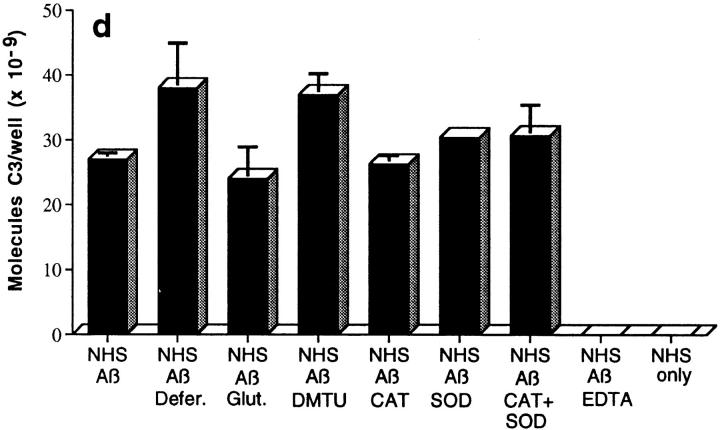
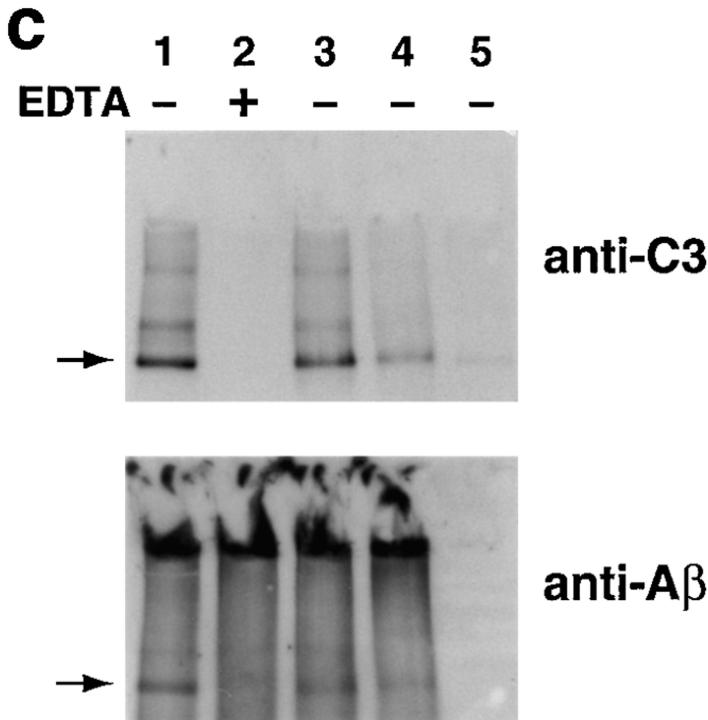
Figure 2.
Assessment of bonds mediating binding of Aβ to C3 activation fragments. (a) Preaggregated Aβ 1–42 (25 μM) was incubated in NHS, and complexes were captured on 10D5-coated wells; NHS only does not contain Aβ. Replicate samples were treated with pH 9.5 buffer, or with the same buffer containing 1 M hydroxylamine (NH2OH ), and remaining bound C3 was then detected and quantitated as described in Materials and Methods. The control containing Aβ, NHS, and EDTA has been subtracted. (b) Replicate wells subjected to treatment with the pH 9.5 buffer or hydroxylamine were evaluated for residual bound Aβ as described in Materials and Methods. (c) Preaggregated Aβ 1–40 was incubated in NHS in the presence or absence of EDTA; lane 5 contains NHS but no Aβ. After centrifugation and washing, fibrillar Aβ pellets were incubated with pH 7.4 buffer (lanes 1 and 2), pH 9.5 buffer (lane 3), or 1 M hydroxylamine in pH 9.5 buffer (lane 4). After further washing, samples were subjected to SDS-PAGE under nonreducing conditions followed by blotting for the presence of C3 and, after stripping, for Aβ. Arrow, The C3 band at ∼180 kD. (d) Preaggregated Aβ 1–42 (50 μM) was incubated in NHS alone, and in the presence of deferoxamine (Defer.; 1 mM), glutathione (Glut.; 1 mM), dimethylthiourea (DMTU; 30 mM), catalase (CAT; 2 × 104 U/ml), SOD (10 μM), or catalase plus SOD, and the Aβ complexes with C3 activation fragments were then detected as described in Materials and Methods.