Figure 1.
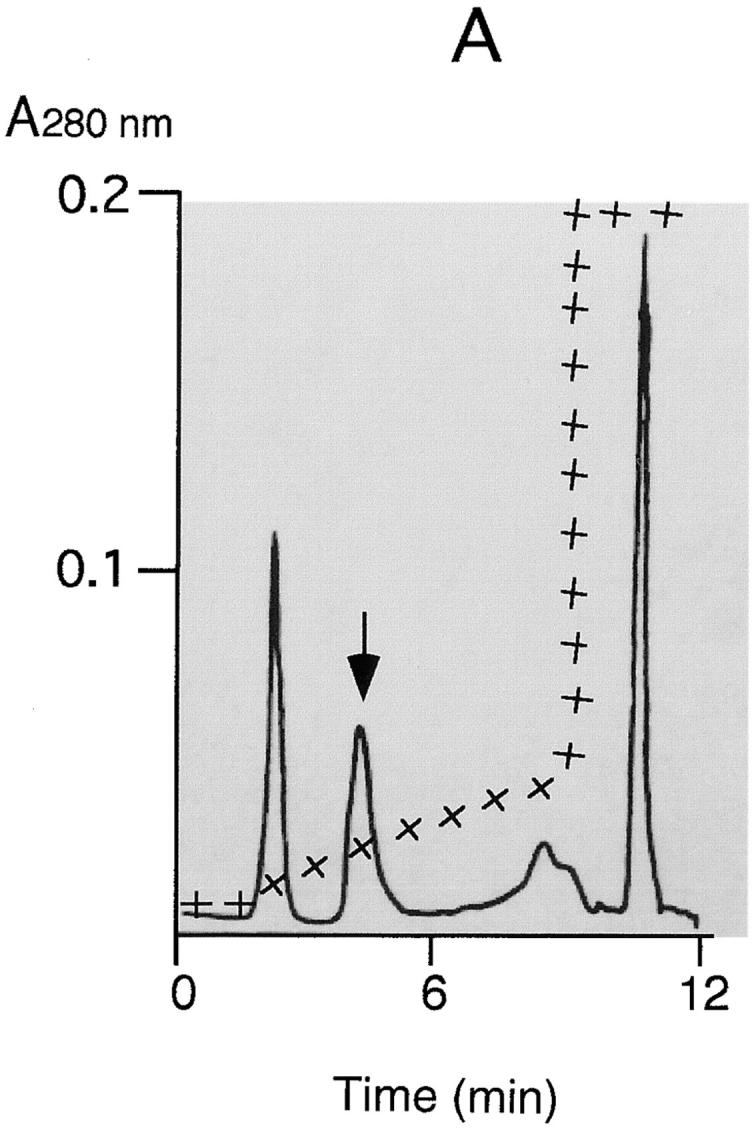
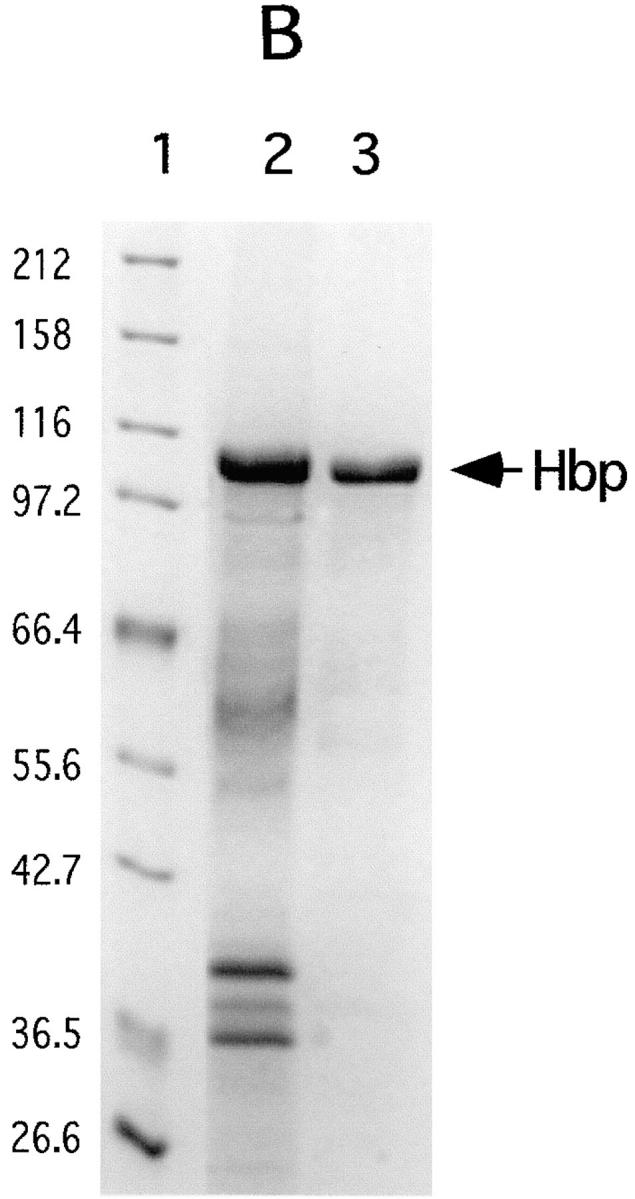
Purification of Hbp. Strain EB1 was cultivated in 5 l MA-medium by gently shaking at 37°C. At an OD660 of 0.6 the culture was cooled to 4°C and the bacteria were separated from the culture supernatant by microfiltration (CFP-2-E-4A membrane cartridge [0.2 μm]; A/G Technology Corporation). The obtained culture supernatant was concentrated a hundred times by using an ultrafiltration column (UFP-10-C-4A membrane cartridge with a NMWC of 10,000; A/G Technology Corporation). A buffer exchange with 50 mM Hepes, pH 7, was done during the concentration step. Six ml of concentrated culture supernatant was then applied to a 0.8-ml POROS 20QE column (PerSeptive Biosystems GmbH, Freiburg, Germany) equilibrated in 50 mM Hepes, pH 7. The column was washed with 8 ml Hepes and developed by a linear salt gradient of 50–200 mM KCl in Hepes. The flow rate during the runs was 4 ml/min. 1-ml fractions were collected and analyzed by SDS-PAGE. (A) POROS 20QE chromatography: elution profile of Hbp. The arrow indicates the Hbp peak. (+) indicates the concentration of KCl during the run (50–1,000 mM). (B) SDS-PAGE profile (11% wt/vol) of samples taken at the two steps of the purification process: the samples were TCA precipitated and analyzed by SDS-PAGE and Coomassie R-250 staining. Lane 1, molecular mass markers (kD); lane 2, the hundred times concentrated culture supernatant (3.5 μg); lane 3, purified 110-kD protein (2.5 μg).
