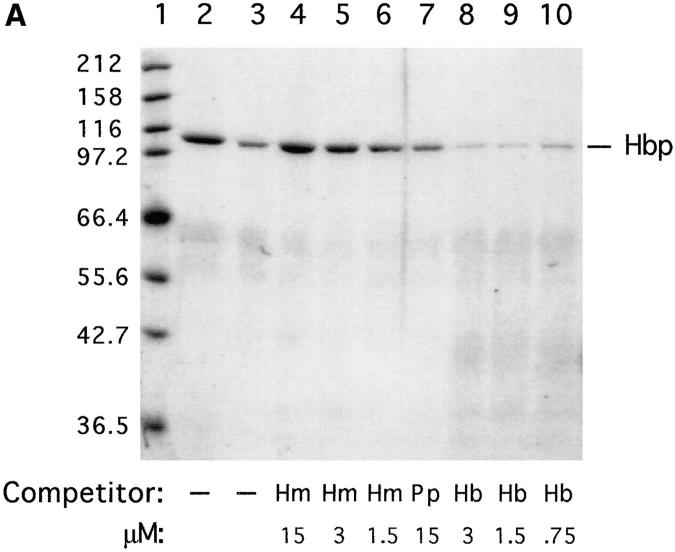
Figure 5.
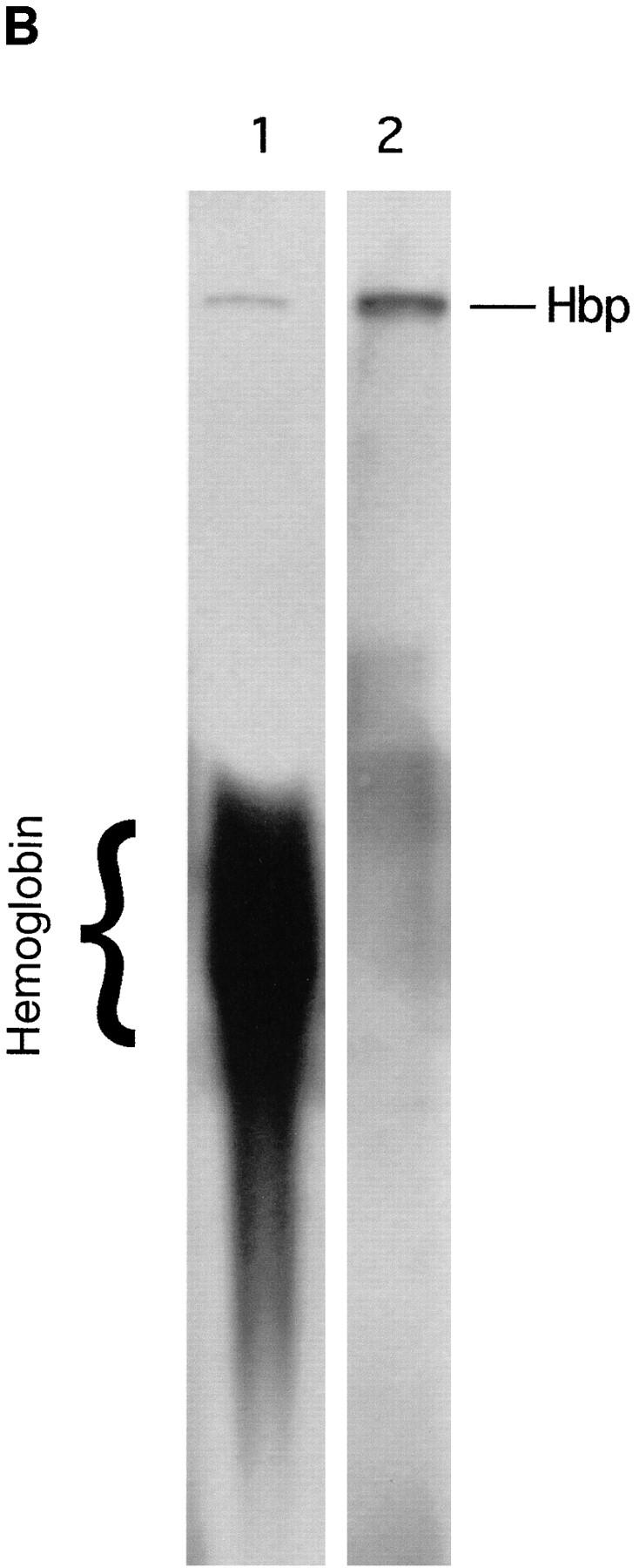
Heme-binding properties of Hbp. (A) Isolation of Hbp by hemin affinity chromatography and binding specificity as measured by competition experiments. 50 ml of culture supernatant of strain DH5α- (pHE12.6) was incubated with hemin-agarose, as described in Materials and Methods. Lane 1, marker proteins (in kD); lane 2, Hbp not retained on hemin-agarose; lane 3, Hbp retained on hemin-agarose alone; lanes 4–6, Hbp retained in the presence of 15, 3 and 1.5 μM hemin (Hm); lane 7, Hbp retained in the presence of 15 μM protoporphyrin IX (Pp); lanes 8–10, Hbp retained in the presence of 3.0, 1.5, and 0.75 μM hemoglobin. (B) Detection of heme-protein complexes in a nondenaturing polyacrylamide gel system. Concentrated supernatant proteins of strain EB1 were incubated with hemin or hemoglobin for 1 h. Samples of these mixtures were run on a 11% polyacrylamide gel. Heme-associated protein bands in the gel were visualized by means of chemiluminescence as described in Materials and Methods. Lane 1, supernatant proteins incubated with hemoglobin; lane 2, supernatant proteins incubated with hemin. The positions of heme-saturated Hbp and hemoglobin in the gel are indicated.