TABLE 1.
Rate constants (25°C) and activation energies
| frq+ | Frq1 | Frq7 | frqS513I | |
|---|---|---|---|---|
| k3, h−1 | 0.05 | 0.15 | 0.05 | 0.05 |
| k5, h−1 (31)* | 0.27 | 0.4 | 0.15 | 0.1 |
| K6, h−1 | 0.07 | 0.1 | 0.01 | 0.006 |
Other rate constants (frq mutant independent; experimental rate constants are indicated by an asterisk followed by a reference): k1 = 1.8 a.u. h−1; k2 = 1.8 a.u. h−1; *k4 = 0.23 h−1 (29); k7 = 0.16 a.u. h−1; k8 = 0.8 a.u. h−1; k9 = 40.0 a.u. h−1; *k10 = 0.1 h−1 (20); k11 = 0.05 h−1; k12 = 0.02 h−1; k13 = 50.0 a.u. h−1; k14 = 1.0 h−1; k15 = 5.0 h−1; K = 1 .25 a.u (describing frq+); K = 8.5 a.u (describing ER24); K2 = 1.0 a.u.; a.u. = arbitrary units.
Activation energies (kJ/mol) for the temperature description of frq+ and ER24: E1 = 62.6; E2 = 20.9; E3 = 25.4; E4 = 15.2; E5, curved Arrhenius equation, see Figs. S4 (Supplementary Material); E6 = 31.9; E7 = 104; E8 = 22.0; E9 = 66.2; E10 = 30.0; E11 = 50.6; E12 = 25.4; E13 = 58.6; E14 = 50.2; E15 = 50.4; EK, curved Arrhenius equation, see Fig. S4 (Supplementary Material); 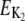 = 68.8.
= 68.8.
