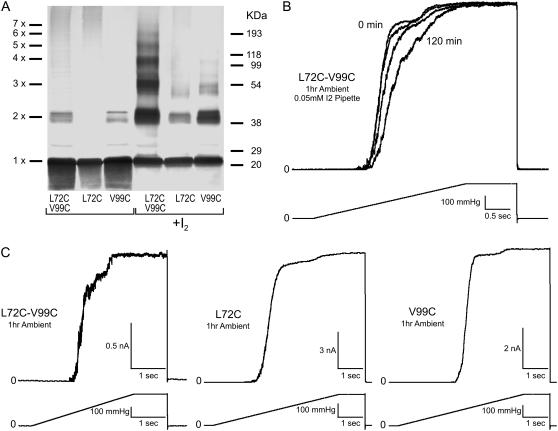
FIGURE 6.
Effects of disulfide cross-link formation in L72C-V99C. Multimerization patterns and functional behavior of mutant channels in patch-clamp. This pair is predicted to link TM2 to TM3 of adjacent subunits. (A) Western blot indicating cross-linking products in the double cysteine mutant separated alongside the products generated in single cysteine controls L72C and V99C under ambient conditions (unmarked) or by adding 0.03 mM iodine (marked +I2). (B) The effect of adding iodine (0.05 mM) from the patch pipette (periplasm) on the activity of the L72C-V99C mutant. Spheroplasts were preincubated under ambient conditions for 1 h. The data show a strong rightward shift of the activation curves upon exposure to I2 signifying channel modification by disulfide formation. Current traces were normalized to the maximal observed current to emphasize the pressure shift (see text for details). (C) Activation of the double cysteine mutant and the single cysteine controls (L72C and V99C) by saturating pressure ramps under ambient conditions. Double mutant activation is heterogeneous, displaying at least two populations of channels. Activities of the controls are like WT in agreement with low disulfide formation under ambient conditions shown in Western analysis.