Figure 7.
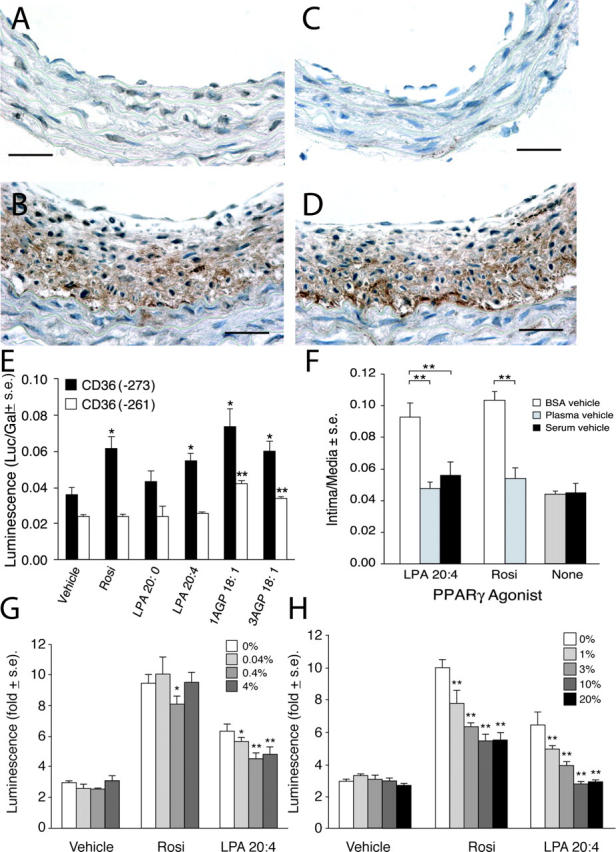
(A) Only a few nuclei show PPARγ immunoreactivity (in a carotid artery 4 wk after treatment with 2.5 μM LPA 20:0). In contrast, the multilayered neointima elicited by LPA 20:4 (B) expresses high levels of PPARγ immunoreactivity. Bars, 250 μm. Activation of PPARγ within neointima in LPA 20:4-treated carotid arteries is indicated by the strong expression of CD36 in a distribution that overlaps that of PPARγ (D). Little immunoreactivity for CD36 was noted in LPA 20:0-treated animals (C). Anti-PPARγ and anti-CD36 were from Santa Cruz Biotechnology, Inc. (E) Stimulation of CD36(−271)-Rluc and CD36(−261)-Rluc reporter genes by Rosi, LPA, and AGP in CV-1 cells. Rosi and LPA 20:4 but not LPA 20:0 (all 10 μM) elicited significant stimulation of CD36(−273)-Rluc that contains a PPRE between bp −273 and −261. Neither compound caused stimulation of the CD36(−261)-Rluc. 1AGP showed higher stimulation compared with 3AGP of the expression of the Rluc reporter. (F) Plasma and serum factors inhibit Rosi- and LPA 20:4-induced neointimal lesion formation in rat carotid arteries. Rosi (10 μM) and LPA 20:4 (2.5 μM) elicited neointima formation 2 wk after treatment when delivered as BSA complexes. In contrast, when the compounds were delivered in rat plasma or serum no neointima formation was detected (n = 5). Effect of BSA (G) or serum (H) on PPARγ activation by CPA and Rosi.
