Figure 3.
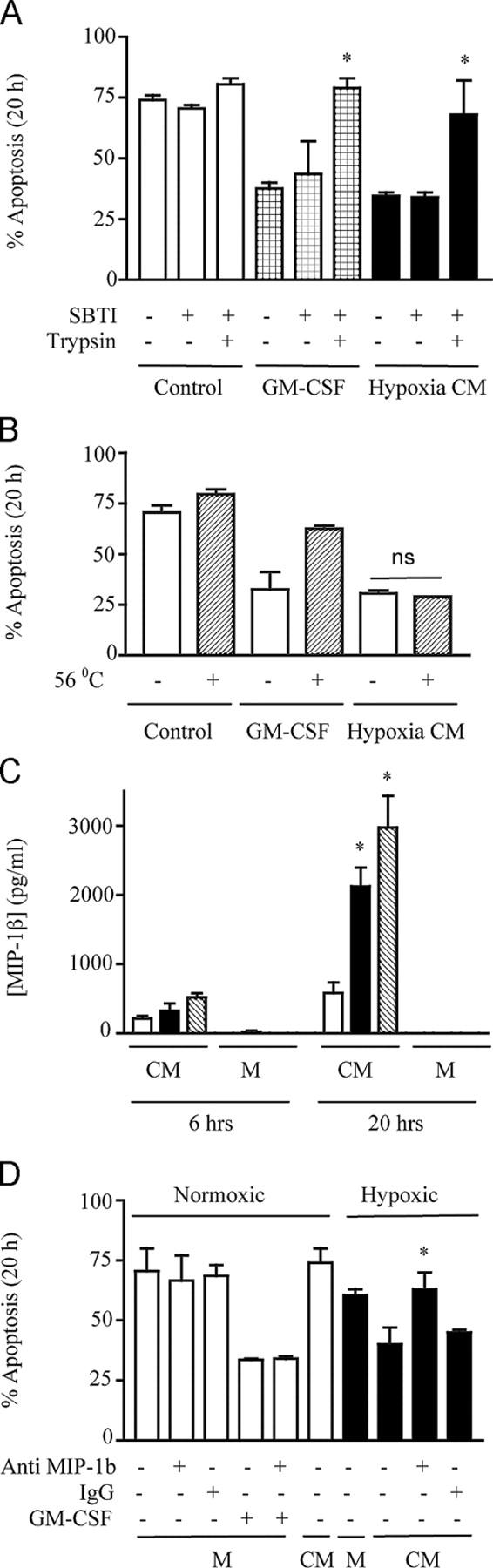
MIP-1β is a novel hypoxia-stimulated granulocyte survival factor. (A) Trypsin sensitivity. Freshly isolated cells were incubated in conditioned medium (CM) obtained from normoxic neutrophils (control) or hypoxic neutrophils (hypoxia CM). These media were either untreated or treated for 2 h with trypsin (1:250 wt/vol), followed by treatment with soya bean trypsin inhibitor (SBTI) or SBTI alone. The effects of trypsin on GM-CSF (100 ng/ml)–mediated neutrophil survival was examined in parallel. Apoptosis was subsequently analyzed at 20 h, and the results represent mean ± SD (n = 2); *, P < 0.05 compared with matched trypsin-untreated conditions. (B) Heat insensitivity. Neutrophils were incubated with CM obtained from normoxic neutrophils (control), hypoxic neutrophils (hypoxia CM), or GM-CSF (100 ng/ml)–supplemented monofeed that was previously heated to 56°C for 45 min. Apoptosis was subsequently analyzed by morphology at 20 h. Results represent mean ± SEM (n = 3). (C) MIP-1β secretion. MIP-1β released into the CM obtained from normoxic (open bar), hypoxic (shaded bar), or anoxic (hatched bar) neutrophils or unconditioned medium (M) was measured by ELISA at 6 and 20 h. Results represent mean ± SEM (n = 3); *, P < 0.05 compared with time-matched normoxic CM. (D) MIP-1β antibody blocks transferable survival. CM obtained from normoxic or hypoxic neutrophils or medium alone (M) was incubated in the presence (+) or absence (−) of 100 μg/ml anti–MIP-1β antibody or 100 μg/ml of total goat IgG isotype control for 30 min at room temperature, before being added to freshly isolated cells. GM-CSF (100 ng/ml) in the presence (+) or absence (−) of anti–MIP-1β or IgG controls were run in parallel, and apoptosis was assessed by 20-h morphology. Results represent mean ± SD (n = 2); *, P < 0.05 compared with matched MIP-1β antibody–untreated conditions.
