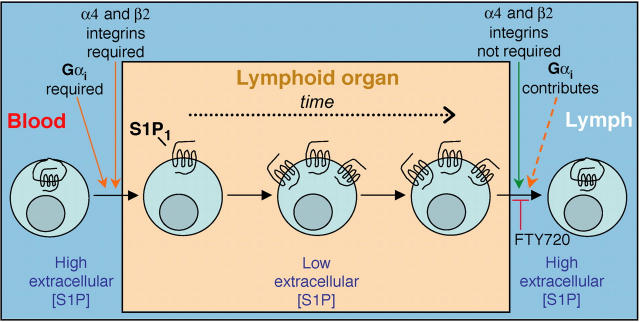
Figure 6.
Summary model relating requirements for lymph node egress and possible effect of cyclical S1P1 expression on lymphocyte recirculation. Lymphocytes in the blood, which has abundant extracellular S1P, have little surface S1P1. After migration into a lymphoid organ in an integrin- and Gαi-dependent manner, the cells begin to up-regulate S1P1, perhaps because extracellular S1P concentrations are no longer adequate to cause receptor down-modulation. Only when S1P1 is up-regulated do the cells become fully egress competent and the time required for up-regulation may help ensure that the cells dwell in the tissue for a period of hours. Additional undefined factors are also anticipated to influence lymphoid organ transit time. Egress occurs in a manner that does not require α4 and β2 integrins but Gαi contributes to this process, perhaps acting downstream of S1P1. Exit sites are suggested to have high S1P levels, and S1P1 signaling is essential for egress to occur. By down-modulating S1P1, FTY720 can block the egress step. Once in the lymph, S1P1 is down-regulated, most likely due to the abundance of S1P, and the cycle begins again. Transit through the splenic white pulp is suggested to involve a similar cycle of events except that egress is into the blood. The reduced S1P1 levels on circulating lymphocytes may also help ensure that S1P does not antagonize chemokine signaling required for lymphoid organ entry.