Fig. 1. Ku protein levels respond to SUMO1 expression.
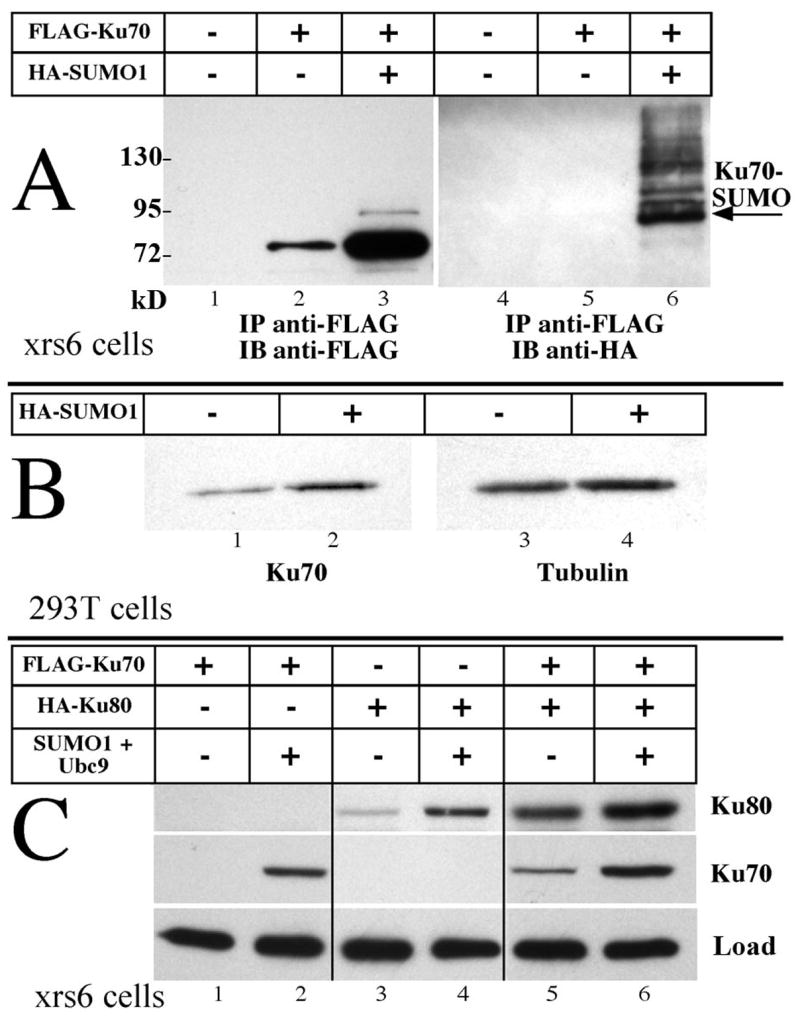
A) Plasmids encoding Ku70 and SUMO1 were variously transfected into CHO.xrs6 cells that lack endogenous Ku80. 500 μg of protein in lysates collected at 48 hours were immunoprecipitated with anti-FLAG agarose, analyzed by SDS-PAGE and visualized with anti-FLAG (lanes 1–3) or anti-HA antibodies (lanes 4–6). The arrow designates the band corresponding to singly SUMOylated Ku70. Unmodified Ku70 increased over five-fold in the presence of additional SUMO1. Protein masses of size markers are indicated in kD. B) Human cell line HEK293T was transfected with plasmids encoding EGFP alone or together with SUMO1. At 48 hours, EGFP positive cells were sorted and harvested. Endogenous Ku70 (lanes 1, 2) and tubulin as loading control (lanes 3, 4) were visualized directly by immunoblotting 1 μg of protein from each lysate. Endogenous Ku70 increased 2–3 folds while tubulin was unaffected. C) Ku70 and Ku80, variously expressed with Ubc9 and SUMO1, show additive effects. Equal loads of 20 μg of protein in each lane were visualized for the two tagged proteins. Lanes from a single gel were assembled for this image. Both Ku70 and Ku80 increase with SUMO1.
