Figure 2.
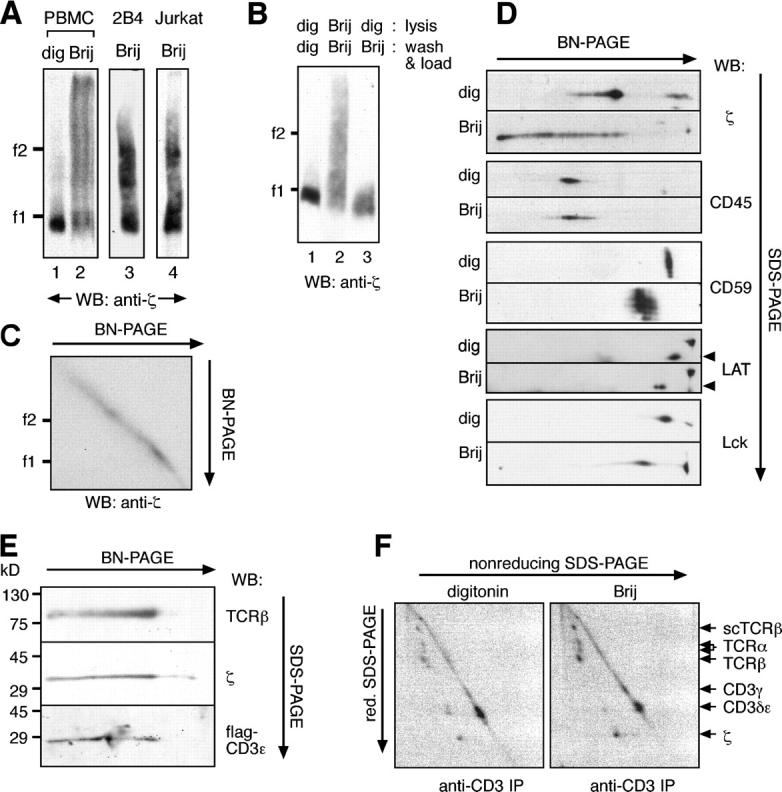
The Brij96-solubilized TCR appears in distinct and stable high molecular weight complexes. (A) Part of the Brij96-solubilized TCR has a low mobility in BN-PAGE. TCRs, prepared from total membranes of human PBMCs (lanes 1 and 2) or by antiphosphotyrosine immunoprecipitation from 2B4 and Jk cells (lanes 3 and 4), were solubilized either in digitonin or in Brij96 and analyzed by BN-PAGE. (B) The TCR does not aggregate in Brij96. Jk cells were lysed, and the TCR was immunoprecipitated with antiphosphotyrosine in the continuous presence of either digitonin (lane 1) or Brij96 (lane 2). Alternatively, they were lysed in digitonin, and immunoprecipitation and loading were performed in Brij96 (lane 3). (C) The TCR does not aggregate during a second BN-PAGE in Brij96. TCRs were isolated from Jk by antiphosphotyrosine immunoprecipitation and analyzed by two-dimensional BN/BN-PAGE. (D) Other cell surface proteins did not appear in high molecular weight complexes when the cells were lysed in Brij96. Total membrane fractions of Jk cells were solubilized in digitonin or Brij96 and separated by two-dimensional BN/SDS-PAGE. Immunoblotting was performed with the antibodies indicated. The arrowheads indicate the LAT spot. (E) The Brij96-extracted TCR contains the α/β, ζ, and CD3 subunits. TCR from Jk.flagɛ cells was analyzed by two-dimensional gel BN/SDS-PAGE. Immunoblotting was performed with antibodies to the indicated TCR subunits. (F) The Brij96-solubilized TCR is not found associated to cell surface proteins other than the TCR subunits. Jk.scTCRβ cells were surface iodinated and lysed in the detergent indicated. After immunoprecipitation with the anti-CD3 antibody UCHT1, TCR complexes were resolved by two-dimensional SDS-PAGE (nonreducing/reducing) and visualized by autoradiography.
