Figure 1.
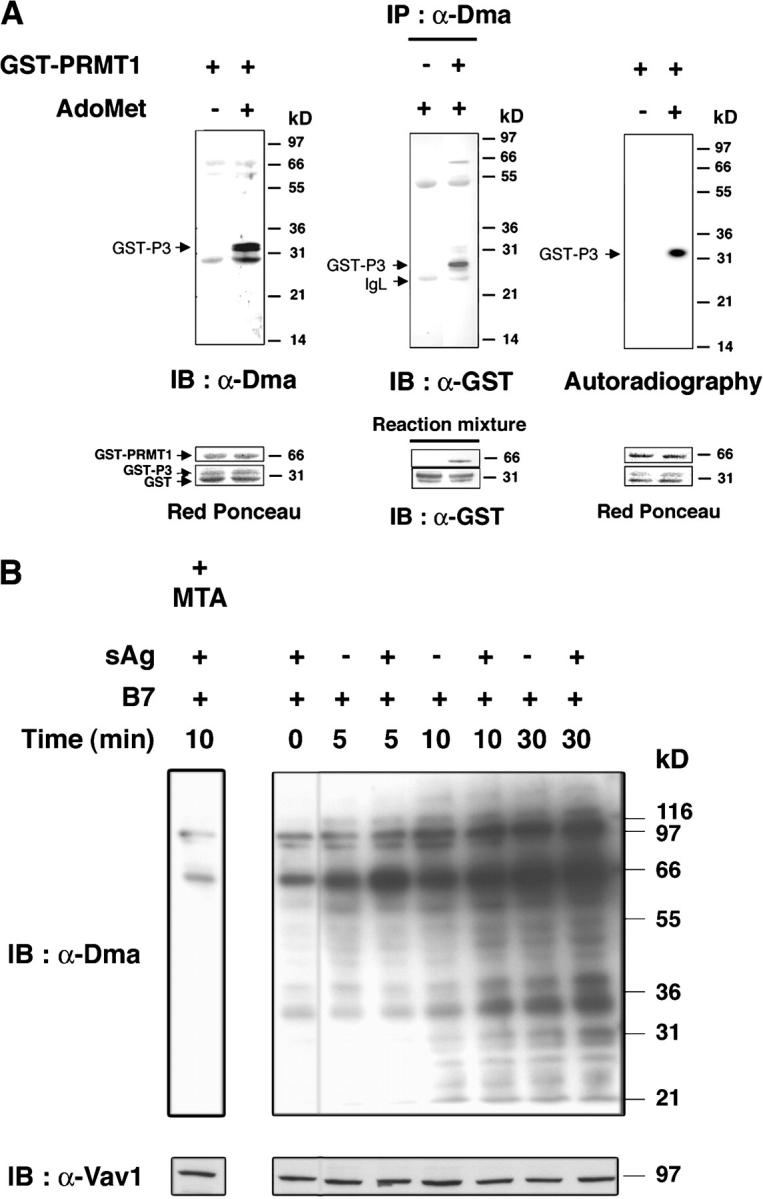
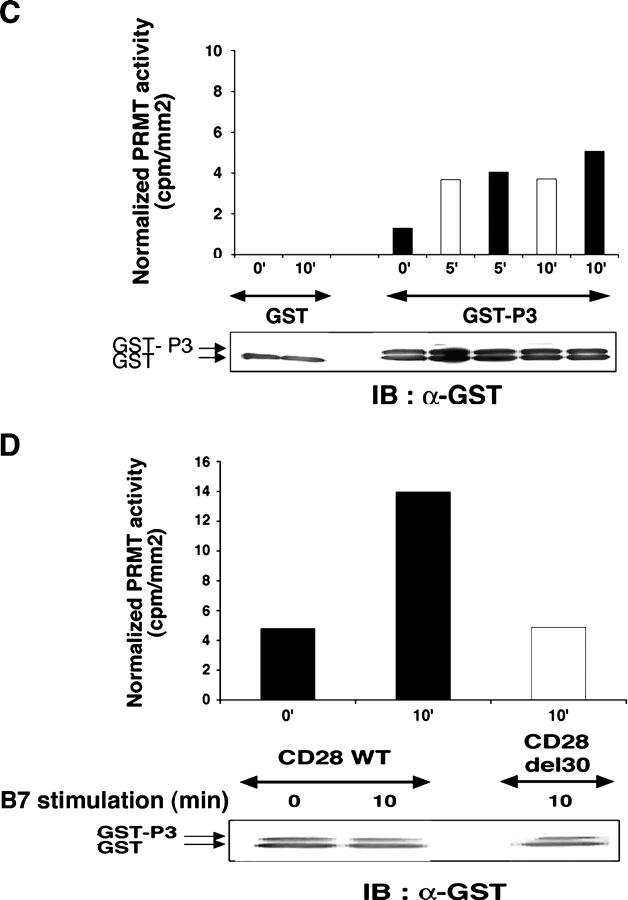
T cell activation increases protein R-methylation and PRMT activity. (A) GST-P3 was reacted with or without AdoMet and GST-PRMT1 (left), or with or without GST-PRMT1 and AdoMet (middle; see Materials and methods) and immunoblotted with α-Dma antibody (left), or immunoprecipitated by α-Dma antibody and then immunoblotted with α-GST antibody (middle). PRMT1-dependent 3H incorporation in P3 in the presence of [3H]-AdoMet (right). Incubation, SDS-PAGE, and blotting were as in the left panel. The apparent discrepancy in migration of GST-P3 between the immunoprecipitate and reaction mixture (middle) was due to the use of NuPAGE Bis-Tris/MOPS and Tris-glycine SDS-gels, respectively. No 3H was detected in GST. Control for reaction mixture is below each experiment. (B) 106 T cells stimulated with 2.5 × 105 5–3.1-B7 cells prepulsed or not with a sAg cocktail (1 μg/ml) for the indicated times as described in Materials and methods. Lysates were immunoblotted with α-Dma (top). Equal loading was controlled by α-Vav1 (bottom). MTA was 0.3 mM for 1 h before stimulation. Shown is one representative of two experiments. (C) PRMT activity was assessed as in A (left) on a lysate of 2 × 106 T cells stimulated as in B with 5–3.1-B7 cells prepulsed (white bars) or not (black bars) with sAg. Substrate content (GST-P3 arrow, bottom) was controlled by α-GST, quantified to normalize radioactivity content (cpm/mm2) associated GST-P3. (D) PRMT activity was detected as in C in lysates of 1.5 × 106 CD28WT (black bars) or CD28Del30 (white bars) cells stimulated 0 or 10 min with 5 × 105 5–3.1-B7 cells. Substrate content was quantified as in C. Data are representative of two experiments.