Figure 3.
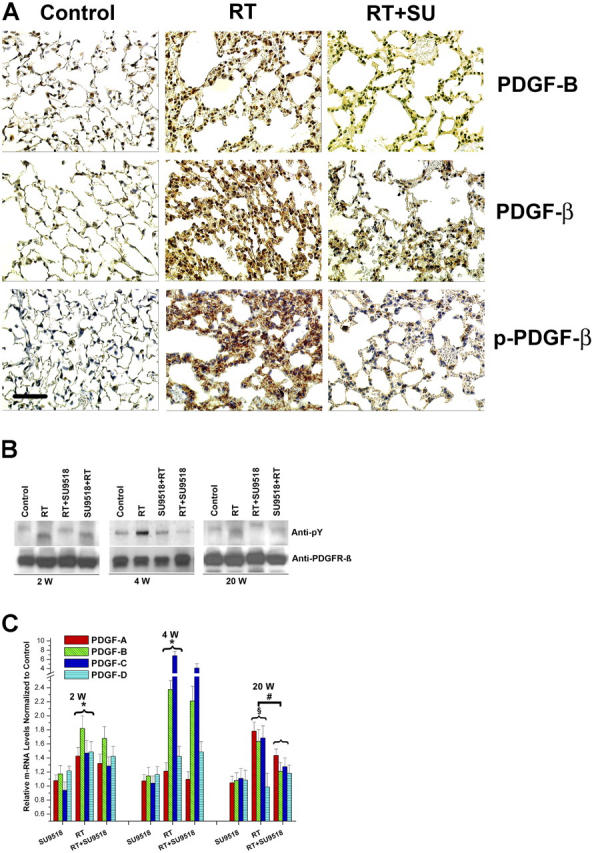
Constitutive activation of PDGF signaling in mouse lungs. (A) Immunohistochemical analysis demonstrates strong induction of PDGF-B and PDGFR-β expression in lung sections 4 wk after 20 Gy total thoracic irradiation (RT). Phosphorylation of PDGFR-β (p-PDGFR-β) was also enhanced in irradiated (RT) versus control mice. Treatment with SU9518 (RT+SU) significantly reduced phosphorylation of PDGFR-β. Bar, 100 μm. (B) 2, 4, and 20 wk after 20 Gy irradiation (RT) and administration of SU9518 (SU+RT and RT+SU), mice were killed and the lungs were harvested for protein isolation. The amount of phosphorylated PDGFR-β (anti-pY) was determined by immunoblotting. Equal loading of lanes was demonstrated with anti-PDGFRβ. (C) Real-time quantitative RT-PCR of PDGF-A, PDGF-B, PDGF-C, and PDGF-D isoforms detected in RNA from mouse lung after SU9518 treatment, 20 Gy thoracic irradiation (RT), and SU9518 administered after lung radiation (RT+SU9518). In irradiated lung specimen, significant up-regulation of all four PDGF isoforms are shown at 2 and 4 wk after irradiation (*, P < 0.01) as well as for PDGF-A, PDGF-B, and PDGF–C isoforms at 20 wk (§, P < 0.001). The expression of the PDGF-A, PDGF-B, and PDGF-C isoforms is significantly impaired at 20 wk after irradiation in SU9518-treated mice (#, P < 0.02), whereas the PDGF expression at earlier time points is not markedly affected by PDGF RTK. This data underscore that the inhibition of fibrosis is the principal target for PDGF RTKIs. Bars are means ± SD from three independent measurements and show relative expression levels compared with the nonirradiated lung tissue at 2, 4, and 20 wk after radiation.
