Figure 1.
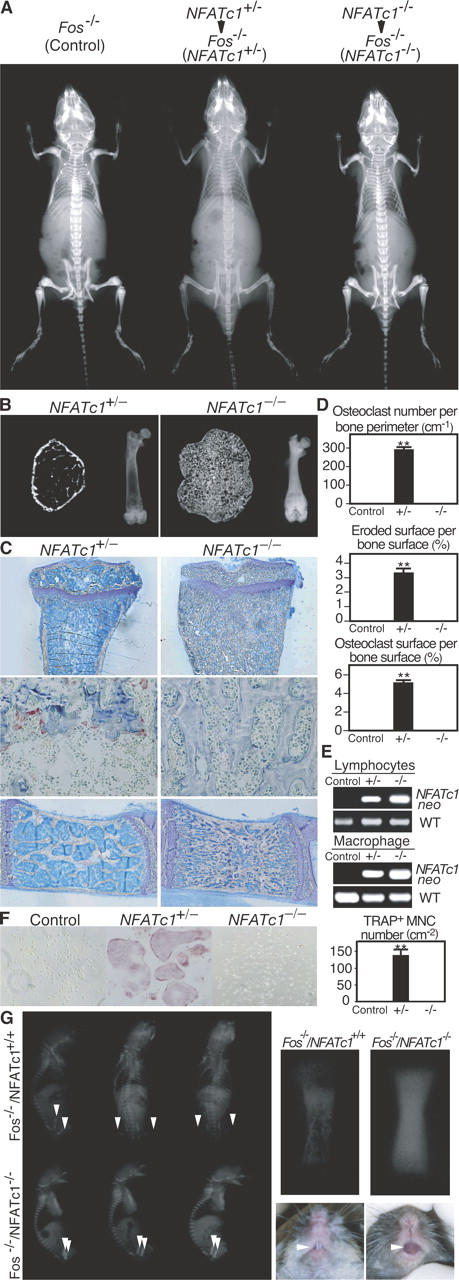
In vivo evidence for the essential role of NFATc1 in osteoclast differentiation. (A) Radiographic analysis of Fos −/− mice transferred with NFATc1 +/− or NFATc1 −/− FLCs. Severe osteopetrosis persists after NFATc1 −/− FLC transfer. (B) Microradiographic analysis of femur (left: microcomputed tomography; right: microradiograph). Bone marrow cavity is filled with unresorbed bone in NFATc1 −/− FLC chimera. (C) Histologic examination of tibia (top: toluidine blue staining; middle: tartrate-resistant acid phosphatase [TRAP] staining for detection of osteoclasts and lumbar vertebra; bottom: toluidine blue staining). No osteoclasts are observed in NFATc1 −/− FLC chimera. (D) Histomorphometric evaluation of osteoclasts in NFATc1 +/− and NFATc1 −/− FLC chimera. (E) Reconstitution of the hematopoietic system of chimeric mice by the donor cells. PCR primers specific for joint region between the NFATc1 locus and the neomycin-resistant gene (NFATc1 neo) and for wild-type (WT) NFATc1 gene are used. The similar chimeric ratios were confirmed by quantitative PCR. (F) Complete blockade of in vitro osteoclastogenesis in osteoclast precursor cells from NFATc1 −/− FLC chimera. Splenocyte-derived osteoclast precursor cells were cultured in RANKL (50 ng ml−1) and M-CSF (10 ng ml−1). We counted multinucleated (>3 nuclei) cells (MNCs) positive for TRAP staining. (G) Radiographic analysis of neonates generated by Fos − / − blastocyst complementation. Note the difference in radio-opacity at the femur (arrowheads). Right panels show the magnified view of the femur of neonates and tooth eruption of 6-wk-old mice. All of the Fos −/−/NFATc1 −/− chimeric mice exhibited osteopetrosis, but osteoclastogenesis is restored in Fos − / − /NFATc1 + / + chimeric mice when ES cells are transmitted to the hematopoietic system of recipient mice. **P < 0.001 versus control.
