Figure 6.
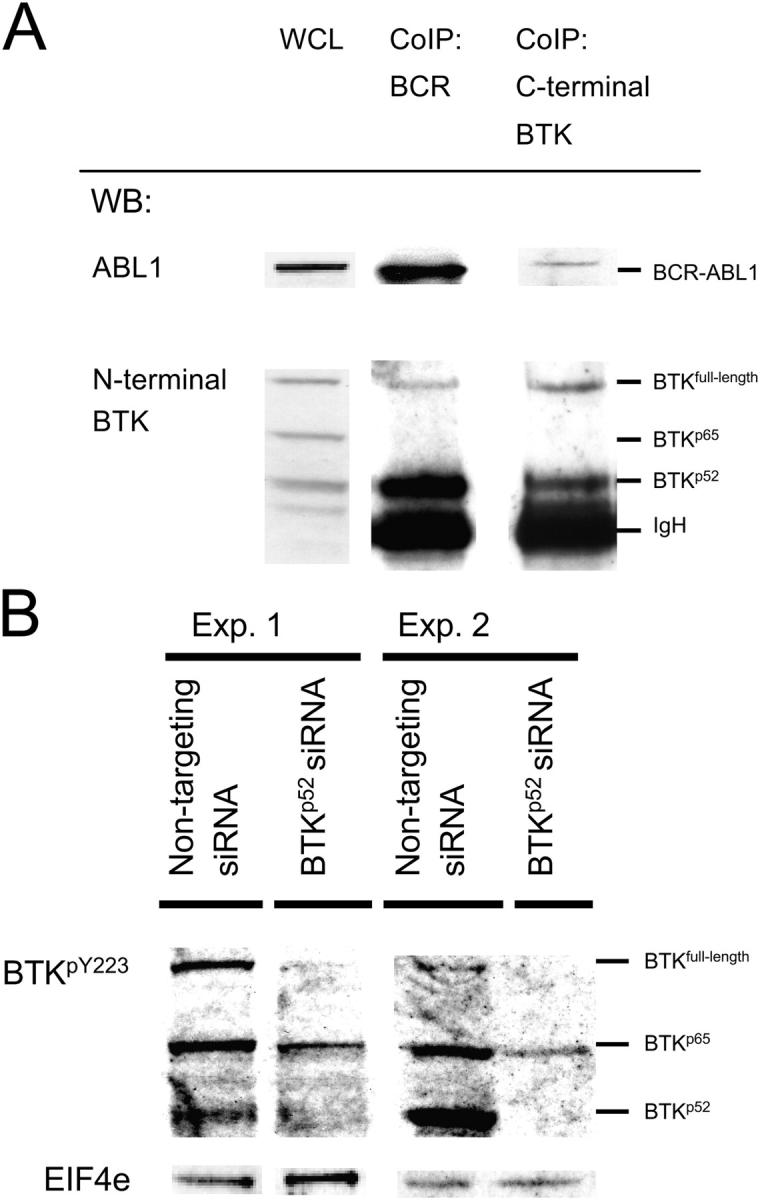
COOH-terminally truncated BTK functions as a linker between full-length BTK and BCR-ABL1. Proteins binding to BCR-ABL1 were coimmunoprecipitated with an anti–BCR antibody (A). Immunoprecipitation was controlled by an anti-ABL1–specific Western blot (WB) showing the characteristic BCR-ABL1 fusion protein expressed by the leukemia cell line. Proteins binding to full-length BTK were coimmunoprecipitated with an antibody against COOH-terminal BTK. Immunoprecipitation was controlled by Western blot using an antibody against NH2-terminal BTK. Full-length BTK and BTKp52 proteins coimmunoprecipitating with BCR-ABL1 or full-length BTK were visualized by Western blot using an antibody against NH2-terminal BTK (A). As a control for quantitative distribution of full-length BTK and BTKp52 before coimmunoprecipitation with BCR-ABL1 or full-length BTK, WCLs were used (A). To analyze the effect of BTKp52 on BCR-ABL1–dependent phosphorylation of full-length BTK, BTKp52 expression was inhibited by RNA interference as described in Fig. 3. As a control, nontargeting siRNA duplices were used. siRNAs were fluorescein-labeled and fluorescein+ cells were sorted and subjected to Western blot using antibodies specific for tyrosine-phosphorylated BTKY223 (B). Because patterns of tyrosine phosphorylation varied between replicate experiments, Western blots of two representative experiments (Exp. 1 and 2) are shown. EIF4e was used as a loading control.
