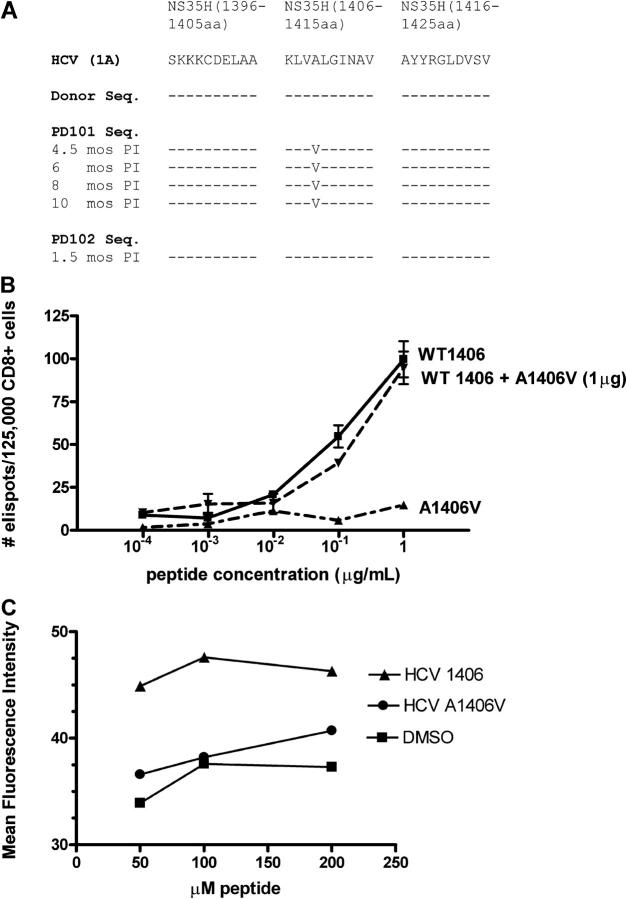
Figure 4.
Mutant viral sequence, recognition, and binding of mutant peptide. (A) Viral sequences corresponding to the single peptide that elicited CD8+ T cells from PD101 who developed viral persistence. The prototype HCV-1 peptide sequence is shown; dashes indicate identity with the prototype and substitutions are represented by standard letter codes at different time points after infection. The donor sequence is identical to the prototype sequence and the sequence derived from PD102 who spontaneously cleared HCV RNA. In contrast, PD101 developed a mutation within the NS3 1406–1415 epitope (A1409V) by 4.5 mo after infection that was propagated until antiviral therapy was initiated. (B) CD8+ T cells from PD101 (from 8 mo after infection) recognize wild-type NS3 1406 peptide, but not mutant 1406 peptide. Dose titrations of wild-type, mutant, and combination of both peptides reveal high frequency of IFN-γ–producing effector cells after pulsing of autologous DCs with wild-type peptide and culture with bead-purified CD8+ T cells without antagonism by the mutant peptide. Wild-type and nonwild-type peptides were screened at multiple concentrations. (C) MHC class I binding affinity. Transporter associtated with antigen-processing–deficient T2 cells (105) were cultured for 16 h at 26°C to increase expression of peptide-receptive cell surface molecules, and then were incubated with various concentrations of individual peptides (wild-type NS3 1406, mutant NS3 1406, or DMSO) at 37°C for 1 h, washed, and stained with FITC-labeled anti–HLA-A2 antibody (BD Biosciences), as described previously (24). The variant peptide bound to the HLA-A2 with decreased affinity (P = 0.0014), comparable to DMSO (no peptide) control. Data are expressed as the mean fluorescence intensity.