Figure 8.
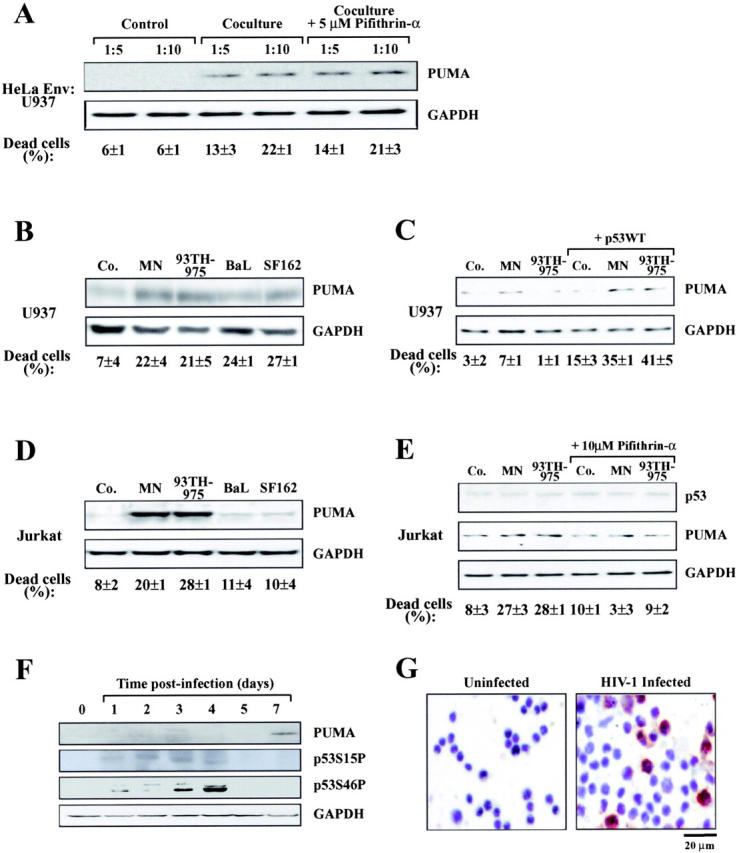
Induction of Puma by Env and HIV-1 in vitro. (A) Induction of Puma in U937 cells cocultured with HeLa Env cells. U937 cells were mixed at different ratios with HeLa Env cells just before the preparation of cell lysates for immunoblot detection of Puma (Control) or coculture for 36 h in the absence or presence of the p53 inhibitor cyclic pifithrin-α (5 μM). The percentage of dead U937 cells was determined by trypan blue exclusion. (B) Induction of Puma by recombinant gp120 protein in U937 cells. U937 were exposed to the indicated gp120 protein (at 500 ng/ml) and cell death, Puma, and GAPDH expression were determined after 4 d. (C) Cooperation between gp120 and p53 to induce Puma in U937 cells. Cells were mock transfected or transfected with wild-type p53. 1 d later, the indicated gp120 proteins were added, and the expression of 53 and Puma were determined 48 h later. (D) Induction of Puma by recombinant gp120 protein in Jurkat cells as in B. (E) Effect of cyclic pifithrin-α. Jurkat cells were treated for 4 d with 500 ng/ml gp120 protein and/or 10 μM cyclic pifithrin-α, followed by determination of Puma expression. (F) Induction of Puma by infection of primary lymphoblasts in vitro. CD4+ lymphoblasts from a healthy donor were infected with HIV-1LAI/IIIb for the indicated period, and proteins (40 μg/lane) were subjected to immunoblot determination of p53 phosphorylation and Puma expression. (G) Puma induction in CD4+ lymphoblasts from a healthy donor infected with a clinical HIV-1 isolate. 5 d after infection, cells were subjected to immunohistochemical detection of Puma. Uninfected cells served as a negative control. Results typical for five independent experiments are shown.
