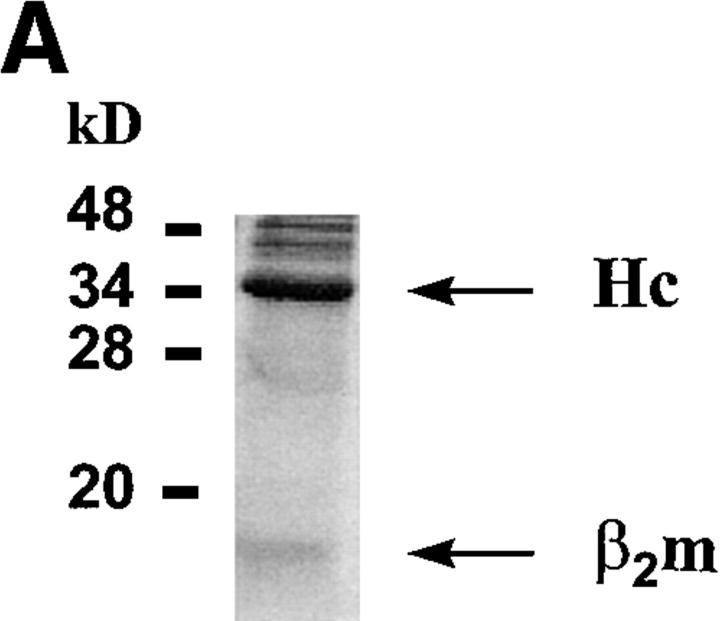
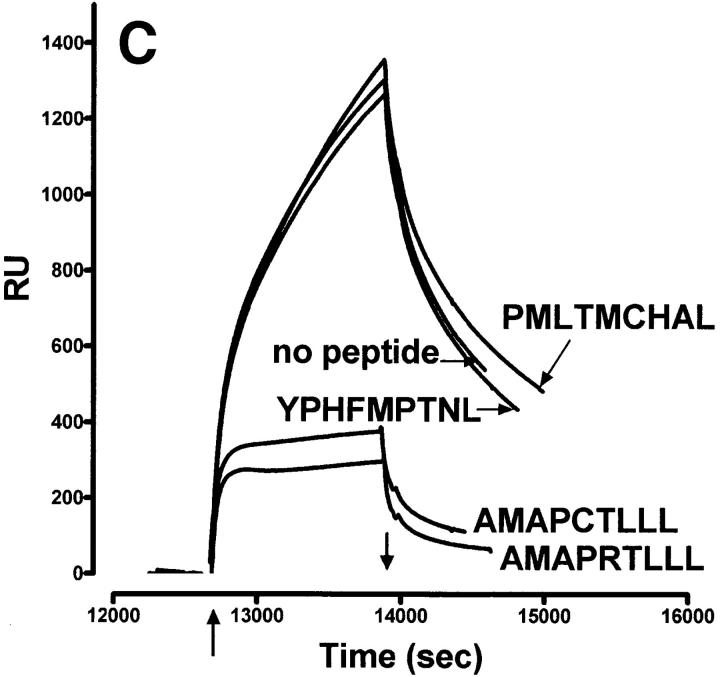
Figure 1.
Characteristics of sQa-1b/β2m secreted from transfected D. melanogaster cells and its specific binding to immobilized QdmC5 peptide. (A) After concentration and purification, the supernatant from Qa-1b/β2m-transfected Drosophila cells was resolved on a 15% SDS-PAGE, and stained using Coomassie brilliant blue. Arrows, Qa-1b heavy chain (Hc) and β2m. (B–D) SPR demonstrating binding of sQa-1b/β2m to immobilized QdmC5. Sensorgrams were obtained using injection volumes of 20 μl at a rate of 1 μl/min. Mass increase due to macromolecular binding is measured in resonance units (RU). Arrowheads, Start (↑ ) and end (↓ ) of the injection. (B) Injection of 0.5 μM sQa-1b or sM3. (C) 0.5 μM sQa-1b was run over the chip alone, or in the presence of 20 μM Qdm (AMAPRTLLL), QdmC5 (AMAPCTLLL), or two control peptides, PMLTMCHAL and YPHFMPTNL. (D) 0.5 μM sQa-1b was run at 1 μl/min for 20 min over immobilized QdmC5 in the absence or presence of competitor peptides. Results are presented as relative binding of sQa-1b, where 0 represents binding in the presence of 20 μM Qdm (25 RU), and 1 is the binding in the absence of peptide (263 RU). *, +, and O represent binding in the presence of 20 μM of the entire 24-mer Dd leader, MGAMAPRTL and MAPRTLLLL, respectively.