Figure 1.
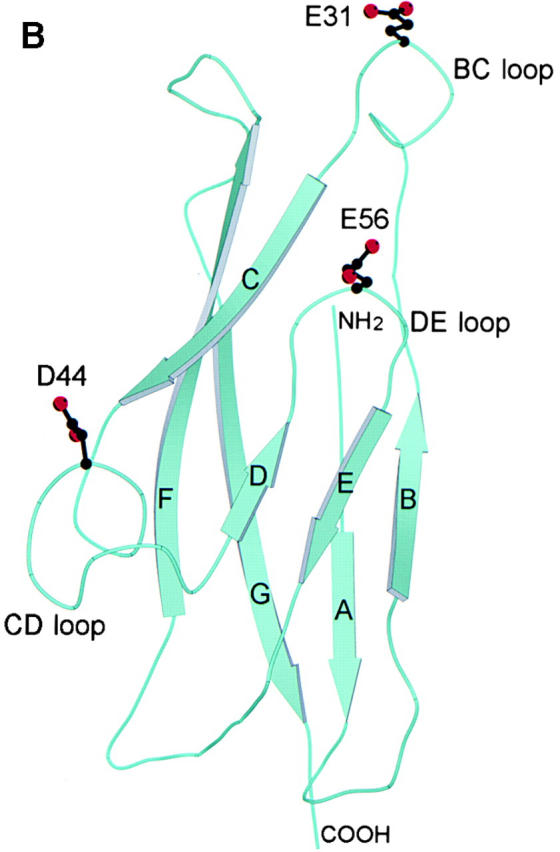
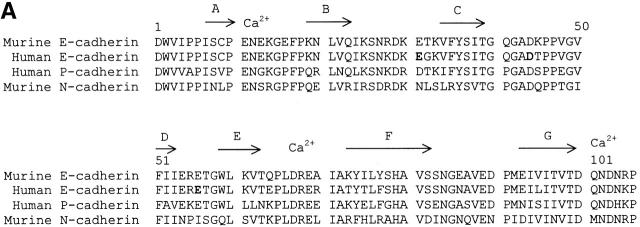
Structural analysis of human E-cadherin. (A) Amino acid alignment of domain 1 of human E-cadherin with human P-cadherin and murine E- and N-cadherin. The positions of the β strands were determined by the Definition of Secondary Structure of Proteins program (reference 51) and are indicated by arrows above the alignment. The PENE sequence in the AB loop, the LDRE sequence in the EF loop, and the DQNDN sequence in the connecting strand, which are predicted to be involved in the binding calcium, are indicated by Ca2+. Residues E31, D44, and E56 are shown in boldface. (B) Ribbon diagram of the human E-cadherin domain 1 model. Human E-cadherin was modeled based on the previously described murine E-cadherin crystal structure. Domain 1 of E-cadherin has seven β strands labeled A through G. The side chains of E31 on the BC loop at the top of domain 1, of D44 on the CD loop on the lower side of domain 1, and of E56 on the DE loop pointing upward in the center of the domain are represented as stick diagrams with carbon atoms, shown in black, and oxygen atoms, shown in red.