Figure 5.
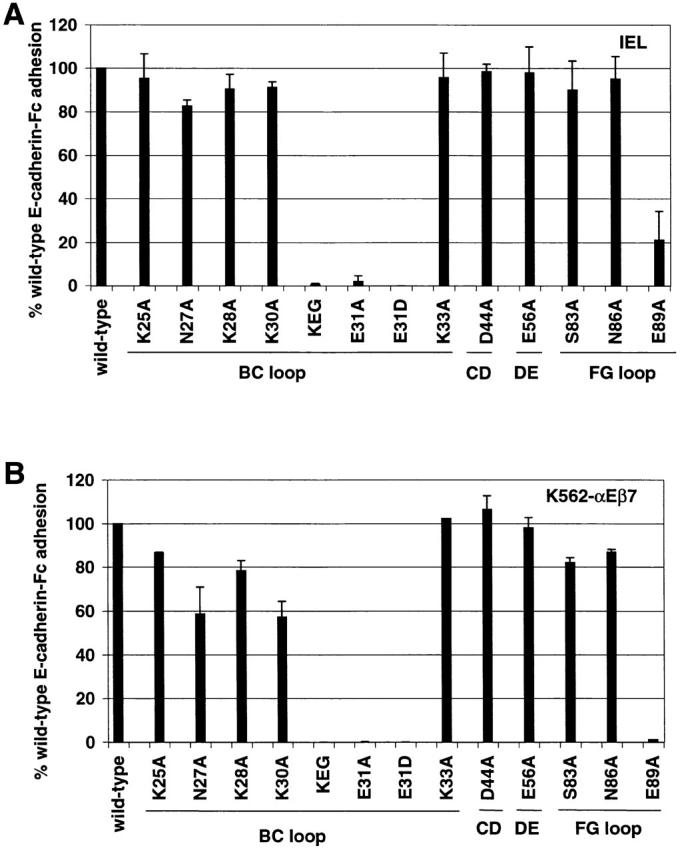
Heterophilic adhesion of IELs (A) and K562-αEβ7 (B) to E-cadherin-Fc mutants. Using adhesion to saturating amounts of E-cadherin-Fc, the mean adhesion to each mutant is expressed relative to adhesion to wild-type E-cadherin-Fc (% cells bound to mutant E-cadherin-Fc/% cells bound to wild-type E-cadherin-Fc × 100). Adhesion to wild-type E-cadherin-Fc is shown as 100%. Adhesion above the saturating dose was chosen to minimize the effect of any errors in quantitation of the fusion protein. Mutation KEG30-32RDT is abbreviated KEG. The loop containing the mutated residues is marked below the lines. Results are shown as the mean ± 1 SD of at least two independent experiments in which each condition was performed in triplicate. A two-sided t test was used to compare the mean value of IEL adhesion to each E-cadherin-Fc mutant with the mean value of IEL adhesion to wild-type E-cadherin-Fc. (A) IEL adhesion to the panel of E-cadherin-Fc mutants. Statistically significant differences are observed for KEG30-32RDT (P ≤ 0.0001), E31A (P ≤ 0.0001), E31D (P ≤ 0.0001), and E89A (P ≤ 0.0001). (B) K562-αEβ7 adhesion to the section of E-cadherin-Fc mutants. Statistically significant differences are observed for N27A (P ≤ 0.0001), K28A (P ≤ 0.0001), K30A (P ≤ 0.0001), KEG30-32RDT (P ≤ 0.0001), E31A (P ≤ 0.0001), E31D (P ≤ 0.0001), N86A (P ≤ 0.0017), and E89A (P ≤ 0.0001).
