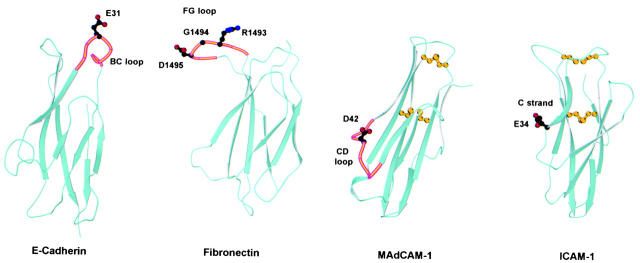
Figure 9.
Comparison of integrin recognition sites in E-cadherin, fibronectin, MAdCAM-1, and ICAM-1. Ribbon diagrams of domain 1 of murine E-cadherin (Protein Database accession no. 1EDH; reference 19), the tenth type III repeat of human fibronectin (accession no. 1FNF; reference 36), domain 1 of human MAdCAM-1 (accession no. 1BQS; reference 10), and domain 1 of human ICAM-1 (accession no. 1IC1; reference 41) are shown. The location of the side chains of acidic residues critical for integrin adhesion are shown with stick diagrams using black for carbon atoms, red for oxygen atoms, and blue for nitrogen atoms. Where applicable, the loop involved in integrin recognition is highlighted in orange. The location of the integrin recognition sites in MAdCAM-1 and ICAM-1 is also representative of VCAM-1 and ICAM-2/ICAM-3, respectively. ICAM-1, ICAM-2, VCAM-1, and MAdCAM-1 all have an extra disulfide bond, not characteristic of the Ig fold, between the BC loop and the FG loop or F strand. The conserved disulfide bond of Ig folds and the extra disulfide bond between the BC and FG loops are shown in yellow in the MAdCAM-1 and ICAM-1 structures.