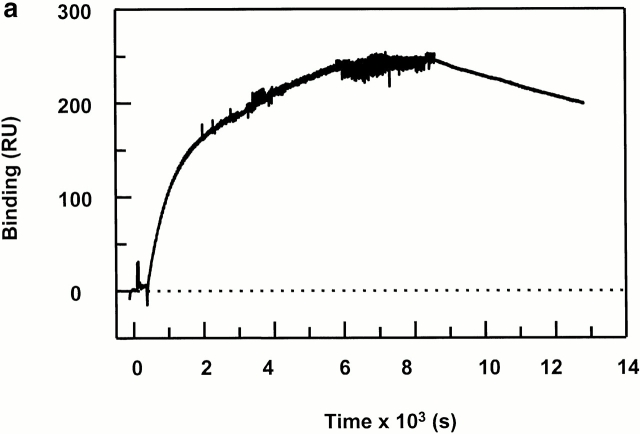
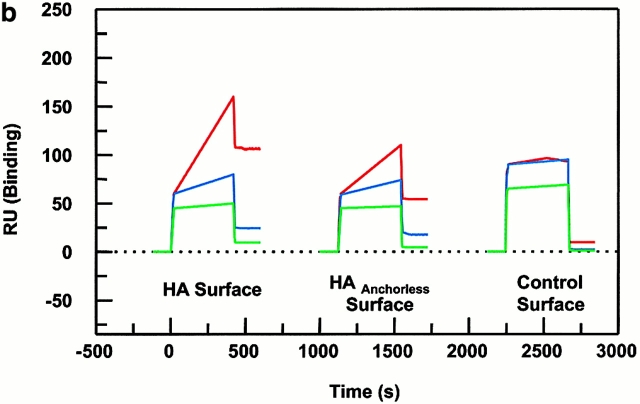
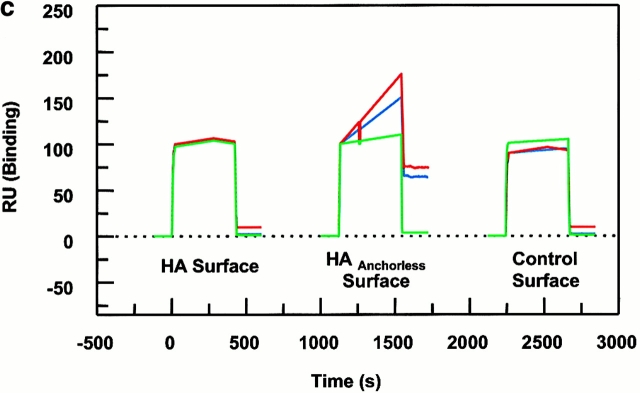
Figure 4.
Peptide binding of DR1 by BIAcore SPR in real time. (a) The binding of DR1βG86Y to immobilized peptide in real time. Cys-HAAnchorless peptide was immobilized in a biosensor flow cell and sensograms were obtained by injection of DR1βG86Y–HAY308A complexes (citrate buffer, pH 6.0). The long-term experiments were performed in a BIAcore X instrument (for details, see Materials and Methods). The binding curve is expressed as RU as a function of time. The DR1βG86Y–HAY308A complexes were prepared by preincubation of DR1βG86Y and HAY308A peptide followed by size-exclusion purification. Running buffer was citrate buffer plus 0.01% Tween 20, pH 6.0. One out of two experiments is shown. (b and c) The effects of DM on binding of the wtDR1 (b) and DR1βG86Y (c) to the peptide surfaces; 2.4 μM wtDR1 or DR1βG86Y, in complex with HAY308A peptide, was isolated by the procedure described in the legend to Fig. 1, and then was incubated at 37°C with or without 1 μM DM for 20 min. Samples (citrate buffer, pH 6.0) were injected over three different peptide surfaces (cys-HA, cys-HAAnchorless, and the control peptide) of BIAcore 2000 at a flow rate of 4 μl/min for 7 min followed by washout (citrate buffer plus 0.01% Tween 20, pH 6.0) at a flow rate of 5 μl/min. Sensograms of DR1 binding are shown in different colors: in the absence of DM (blue), presence of DM (red), and DM plus soluble peptide for blocking (green). One out of three experiments is shown.