Abstract
Over the past decade, many of the key components of the genetic machinery that regulate the asymmetric division of Drosophila melanogaster neural progenitors, neuroblasts, have been identified and their functions elucidated. Studies over the past two years have shown that many of these identified components act to regulate the self-renewal versus differentiation decision and appear to function as tumor suppressors during larval nervous system development. In this paper, we highlight the growing number of molecules that are normally considered to be key regulators of cell cycle events/progression that have recently been shown to impinge on the neuroblast asymmetric division machinery to control asymmetric protein localization and/or the decision to self-renew or differentiate.
The machinery that drives neuroblast asymmetry and the differential fate of the daughters
One of the best Drosophila melanogaster models for studying asymmetric division are the neural progenitors, or neuroblasts, which go on to generate the majority of the cells of the central nervous system. Neuroblasts undergo asymmetric divisions, generating two daughter cells of distinct size and fate. The larger daughter retains neuroblast identity and can continue to divide asymmetrically and self-renew, whereas the smaller daughter, namely the ganglion mother cell (GMC), is committed to the differentiation pathway and divides terminally to produce two neurons or glial cells. Through repeated self-renewing asymmetric divisions, neuroblasts, like other stem or progenitor cells, can generate a large number of differentiated progeny during their lifetime.
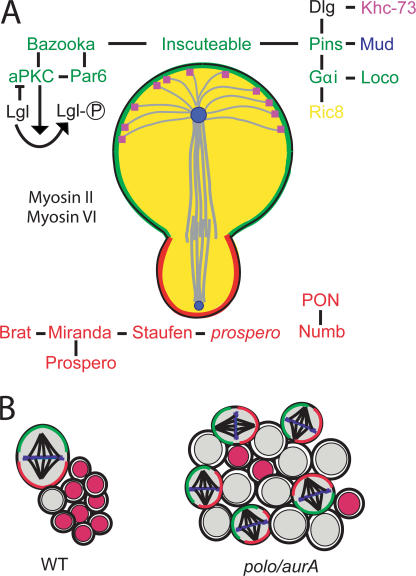
Many key components of the genetic machinery that facilitate the neuroblast asymmetric division have been identified and characterized (Egger et al., 2008; for review see Yu et al., 2006). There are in essence three key features associated with the neuroblast asymmetric division: (1) cell fate determinants, which act as differentiation factors, are asymmetrically localized as cortical crescents during mitosis; (2) the mitotic spindle is oriented orthogonal to the cortical protein crescents to ensure their exclusive segregation to the GMC daughter; and (3) the mitotic spindle is itself asymmetrical, resulting in the production of a larger neuroblast daughter and a smaller GMC daughter. All three features of this asymmetric division appear to be regulated by a set of proteins localized to the apical cell cortex starting during the late G2 phase of the cell cycle. These key components and their roles in mediating the neuroblast asymmetric division are summarized in Fig. 1 A. The cell fate determinants are localized to the basal cell cortex of embryonic neuroblasts, and the mitotic spindle is aligned along the apicobasal axis. A subset of these embryonic neuroblasts become quiescent, and proliferation is reinstated during larval development. The basic machinery involved in the asymmetric division of these larval neuroblasts appears to be conserved with embryonic neuroblasts; however, larval neuroblasts of the central brain divide without a fixed orientation.
Figure 1.
Summary of some of the key players and features of neuroblast asymmetric division. (A) Asymmetrical segregation of basal cell fate determinants specifically into the daughter GMC requires the correct localization of protein complexes to the apical cell cortex. The apically localized proteins comprise two protein complexes linked by the adaptor protein Inscuteable. The evolutionary conserved Par protein cassette comprising Bazooka/Par3, aPKC, and Par6 is the first protein complex to localize to the neuroblast cell cortex and is primarily involved in excluding the basally localized proteins from the apical cortex. This protein cassette regulates the activity of the tumor suppressor lethal giant larvae (Lgl), which is also essential for correct targeting of the basal protein complexes. Par6 can directly associate with Lgl, and it is in this complex that aPKC is believed to inactivate Lgl by phosphorylation. Miranda is thus recruited to the basal cell cortex by the active nonphosphorylated Lgl. The second apical protein complex contains proteins involved with heterotrimeric G protein signaling, including Gαi, Partner of Inscuteable (Pins), and Locomotion defects (Loco). This complex is thought to mediate a receptor-independent heterotrimeric G protein signaling mechanism involving the regulation of Gαi through interactions with the cytoplasmic guanine nucleotide exchange factor (Ric-8) and guanine nucleotide dissociation inhibitors (Loco and Pins). The Gαi–Pins–Loco complex mediates mitotic spindle formation and alignment to ensure that the cleavage plane is orthogonal to the apical/basal polarity axis. The geometry of the neuroblast mitotic spindle is asymmetrical; the spindle length is longer on the apical side, and the entire spindle is displaced toward the basal cortex. The centrosomes are also nonequivalent, with the larger mother centrosome emanating more extensive astral microtubules and being preferentially retained within the neuroblast through subsequent divisions. Pins can also associate with the centrosome- and apical cortex–associated nuclear mitotic apparatus protein–-related protein mushroom body defective (Mud), which is essential for proper spindle alignment, as well as Discs large (Dlg) and the astral microtubule plus end protein Khc-73 to induce cortical polarity. The actin/myosin cytoskeleton also plays an important role in the assembly of these apical/basal protein complexes. Actin filaments but not microtubules appear to play an essential role in cortical tethering of the proteins, and the Drosophila myosins II (Zipper) and VI (Jaguar) exist in mutually exclusive complexes with Miranda and are essential for correct asymmetric localization of the cell fate determinants. The basal proteins exist as two protein complexes. One complex contains the adaptor protein Miranda, which associates with and facilitates the asymmetric localization of the translational repressor Brain tumor (Brat), the homeodomain transcription factor Prospero, and the double-stranded RNA-binding protein Staufen, which itself can bind prospero transcripts. The second complex contains the Notch antagonist Numb and its binding partner Partner of Numb (Pon). Upon segregation into the GMC, Miranda is degraded, allowing Prospero to translocate into the nucleus to activate genes involved in differentiation and repress genes involved in proliferation. The GMC divides terminally to produce two neurons or glia. Note that the apical/basal nomenclature is based on embryonic neuroblasts and that neuroblasts in the central brain divide without a fixed orientation. Please note that the color of the lettering corresponds to the protein's localization in the schematic picture; in the case of black lettering, the protein can be found throughout the cortex. (B) Postembryonic neuroblasts divide to produce a lineage of differentiated progeny. The cell types of the lineage can readily be distinguished with neuroblast markers such as Insc (green), Miranda (red), and Deadpan (gray) and markers for differentiated progeny like Elav and nuclear Prospero (red). A disruption to cell polarity and/or spindle orientation (e.g., in aurora A and polo mutants) can affect the balance between self-renewal and differentiation, resulting in too many self-renewing cells at the expense of differentiated progeny. WT, wild type.
Failure in asymmetric division, overproliferation, and tumor formation
The Drosophila larval brain neuroblast has recently emerged as a novel model for the study of stem cell self-renewal and tumorigenesis. Several types of studies have led to the view that defective asymmetric division may lead to the generation of tumors. First, brain tissue mutant for several of the components that control neuroblast asymmetric division (e.g., Miranda, Prospero, Numb, lethal giant larvae [Lgl], Brat, and Partner of Inscuteable [Pins]) will undergo massive overgrowth upon transplantation into the abdomen of wild-type hosts, killing the host within weeks (Caussinus and Gonzalez, 2005; Beaucher et al., 2007). These implanted cells exhibit many of the hallmarks of malignant neoplastic growth. They appear to be immortal and can be serially transplanted into successive hosts over years. They exhibit genome instability as indicated by high frequencies of cytologically abnormal karyotypes as well as defects in centrosome morphology and number. These transplanted cells can also exhibit metastatic behavior, migrating away from the site of the primary tumor, passing through several cell layers, and establishing secondary colonies. Because the tumors derived from tissues mutant for different components of the neuroblast asymmetry machinery are essentially indistinguishable, it seems likely that they arise from a common mechanism: the disruption of neuroblast asymmetry and the production of excess self-renewing cells.
Supporting this view, a second series of recent studies have shown that all of the basal cell fate determinants (Prospero, Brat, and Numb as well as their adaptor molecules Miranda and Partner of Numb; Bello et al., 2006; Betschinger et al., 2006; Choksi et al., 2006; Lee et al., 2006a,c; Wang et al., 2006), which facilitate their asymmetric localization, can act as tumor suppressors (Fig. 1). Larval neuroblasts homozygous for mutations in any of these genes produce supernumerary self-renewing daughters at the expense of differentiated cells. These observations suggest that the loss of or a failure to correctly asymmetrically localize these determinants in larval neuroblasts can result in the failure to correctly specify the fate of their daughters, which can, in turn, lead to overproliferation and tumorigenesis. Consistently, several earlier studies showed that mutations in three genes, discs large (dlg), (lgl), and scribble (scrib), which induced the formation of malignant neoplastic tumors of the nervous system, also caused defects in the asymmetric localization of the cell fate determinants in neuroblasts (Ohshiro et al., 2000; Peng et al., 2000; Betschinger et al., 2006; Lee et al., 2006b). Lgl functions to restrict atypical PKC (aPKC) to the apical daughter (self-renewing cell), and it is also the target of aPKC phosphorylation (Fig. 1; Lee et al., 2006b). Together, these studies suggest a causal link between defects in neuroblast asymmetric division and overproliferation/tumorigenesis in the larval brain. These findings have recently been reviewed and will not be discussed in detail here (for reviews see Yu et al., 2006; Gonzalez, 2007).
Cell cycle genes can regulate asymmetric division and act as tumor suppressors
Recent published and unpublished studies have reinforced an earlier view that cell cycle regulators can impinge on the asymmetric division machinery. Mutations in several genes encoding key regulators of cell cycle events can affect asymmetric protein localization, specification of distinct daughter cell fates, and/or the decision to self-renew or differentiate. In addition, the activation of cell cycle proteins, including CDK1, aurora A, and Polo, at prometaphase and metaphase coincides with the timing of asymmetric protein localization during neuroblast divisions, leading to a delicate temporal control of asymmetric division.
cdc2/CDK1 levels can determine whether a neural progenitor division is symmetric or asymmetric
The first indication that cell cycle regulators might also control aspects of the asymmetric division of neural progenitors came from a study on Cdc2/CDK1 (Tio et al., 2001). A dominant-negative allele of cdc2, cdc2E51Q, was isolated in a genetic screen designed to identify mutations that converted asymmetric GMC divisions that produced two daughter neurons with distinct identities into symmetric divisions generating two neurons of identical fate. Cdc2 in complex with the A- or B-type cyclins provides the kinase activity (CDK1) that is necessary to drive cells from G2 to mitosis, and cells lacking CDK1 activity arrest in G2 phase. Analysis using cdc2E51Q as well as a temperature-sensitive allele of cdc2 under conditions in which the activity of cdc2 was attenuated, but not sufficiently so to prevent cells from entering mitosis, resulted in the failure to asymmetrically localize both the apical and basal components of the neuroblast asymmetry machinery, causing asymmetric divisions to be converted to symmetric divisions. Therefore, it appeared that there exists an intermediate level of cdc2 activity that enabled neural progenitors (and muscle progenitors) to divide but did not allow the division to be asymmetric because of a failure in asymmetric protein localization.
A direct demonstration that cdc2 activity was required during mitosis for asymmetric protein localization was facilitated by the knowledge that asymmetric protein localization does not require intact microtubules. In neuroblasts arrested at prometaphase using a microtubule-depolarizing drug in which all (both maternal and zygotic) of the cdc2 is temperature sensitive, normal apical and basal protein crescents are formed at the permissive temperature. However, after a shift to the nonpermissive temperature, asymmetric protein localization cannot be maintained. If it is CDK1 activity that is responsible for the maintenance of asymmetric protein localization, attenuating cyclin levels might also be expected to cause defects in asymmetric protein localization. Cyclin A is degraded at prometaphase, whereas cyclin B and B3 are degraded during anaphase. In neuroblast double mutants for the late degrading cyclin B and B3, mislocalization of both apical and basal components can be seen at metaphase coinciding temporally with cyclin A degradation. These observations support the view that high levels of CDK1 activity are required during mitosis to maintain asymmetric protein localization and that it is possible to convert an asymmetric division into a symmetric division by altering the levels of CDK1 activity.
Aurora A and Polo kinases act as tumor suppressors by preventing excess self-renewal
Two other highly evolutionally conserved kinases, aurora A and Polo, have recently been shown to impinge on the neuroblast asymmetric division machinery and exhibit tumor suppressor properties in the larval brain (Lee et al., 2006a; Wang et al., 2006, 2007). Both kinases were initially identified as centrosomal proteins that have roles in mediating a multitude of mitotic processes. Loss of function mutations in either gene had previously been described as causing defects in centrosome maturation, delay/arrest at metaphase, or defects during cytokinesis (Llamazares et al., 1991; Glover et al., 1995;Carmena et al., 1998). Surprisingly, however, it was shown recently that mutations in aurora A or polo cause massive overgrowth in the brain but not other tissues (Lee et al., 2006a; Wang et al., 2006, 2007).
Live imaging (for aurora A mutants) and clonal analyses indicate that mutant brain neuroblasts can produce two self-renewing daughters, leading to an excess of neuroblast-like cells at the expense of differentiated neurons. Asymmetric localization of Numb and Pon (but not Prospero, Miranda, and Brat) is adversely affected in the aurora A and polo mutant neuroblasts. Presumably as a result of the partial loss of function, cell division can occur, although asymmetric protein localization is disrupted. Although this defect is one of several (see the next two paragraphs) caused by aurora A and polo mutants, it alone is sufficient to cause the observed overproliferation because clones in the larval central brain derived from single neuroblasts mutant for numb or pon exhibit excess proliferation at the expense of differentiation. Moreover, this overproliferation observed in aurora A and polo mutants can be largely but not completely reversed by overexpressing wild-type Numb. Interestingly, clones derived from single neuroblasts expressing a constitutively activated form of Notch in the central brain also exhibit an overproliferation phenotype similar to that seen in aurora A and numb loss of function. However, Notch is not required for neuroblast proliferation in the ventral nerve cord, suggesting that its role in neuroblast proliferation differs in different tissues (Almeida and Bray, 2005). Attenuating Notch in either aurora A or polo homozygous mutant background can suppress the overproliferation phenotype, albeit partially. These findings suggest that a genetic hierarchy comprising aurora A/polo, numb, and the neuroblast act to ensure that Notch is preferentially activated only in the daughter cell, which adopts progenitor identity where it acts to promote self-renewal.
Little is known about the biochemical substrates through which Aurora A might act to suppress excess proliferation. However, Pon has been shown to be a functionally important downstream target of Polo kinase for the regulation of neuroblast asymmetric division (Wang et al., 2007). Numb asymmetric localization is facilitated by Pon, which is itself asymmetrically localized. The C-terminal localization domain (Pon-LD), which is necessary and sufficient to mediate Pon asymmetric localization, contains a serine residue (S611) that matches the consensus phosphorylation site for Polo. Both in vitro and in vivo experiments suggested that Polo can directly phosphorylate Pon. The significance of this phosphorylation is demonstrated by the fact that Pon S611 phosphorylation is essential for Pon asymmetric localization. Thus, Polo can regulate the asymmetric division of neuroblasts by phosphorylating and, thereby, facilitating the asymmetric localization of Pon. Consistently, Polo is also required for the asymmetric localization of Numb during neuroblast divisions.
These findings illustrate the importance of Numb/Pon as downstream components of aurora A and polo in mediating the asymmetric fates of the neuroblast daughters. However, it is important to emphasize that polo/aurora A loss of function, in addition to impinging on Pon/Numb asymmetric localization, also affects several distinct pathways/components that can also contribute to the self-renewal versus differentiation decision. Neuroblasts mutant for polo/aurora A also fail to asymmetrically localize aPKC, which has properties consistent with that of a proliferation factor. In addition, the tight coupling seen in wild-type neuroblasts, in which the mitotic spindle is always oriented orthogonal to the cortical protein crescents, is disrupted in polo/aurora A mutants. It is known that neuroblasts mutant for components of the centrosome, like centrosomin and mushroom body defect, which disrupt mitotic spindle orientation, can also exhibit overproliferation, although this effect is weak (Bowman et al., 2006; Izumi et al., 2006; Lee et al., 2006a; Siller et al., 2006). During mammalian neurogenesis, spindle orientation has also been shown to be an important determinant for the choice of asymmetric division versus symmetric division. Loss of function of several centrosomal components (i.e., abnormal spindlelike microcephaly associated) results in predominant asymmetric division and premature differentiation of neural progenitors and the formation of a smaller brain (the related disease is termed microcephaly in human patients; Bond et al., 2002). In another study, knockdown of mouse inscuteable expression changed the division plane of neural progenitors and resulted in more frequent symmetric divisions that lead to enhanced proliferation (Zigman et al., 2005). Thus, the phenotype induced by polo/aurora A mutants is not merely caused by disruption of the Numb–Notch pathway but the sum of the effects exerted on multiple pathways. In view of the pleiotrophic nature of these kinases, it is not surprising that although expressing a phosphomimetic form of Pon in polo mutant neuroblasts can restore asymmetric Numb localization, the overproliferation, spindle orientation, and aPKC asymmetric localization defects remain.
The tumor suppressor function of Aurora A and Polo in Drosophila larval brains is in contrast to the previously reported and widely accepted view that they act as oncogenes in mammalian cells (Zhou et al., 1998). Both mammalian Aurora A and Pololike kinase 1 can phosphorylate tumor suppressor p53, leading to its destabilization and degradation, and, thus, appear to act as negative regulators of p53 (Ando et al., 2004; Katayama et al., 2004). Conversely, the overexpression of Aurora A or Polo can induce oncogenic transformation, presumably through down-regulating p53 functions. Overexpression of Aurora A or Pololike kinase 1 can also lead to the generation of multiple centrosomes through defects in cell division and consequent tetraploidization, thereby leading to tumor progression (Meraldi et al., 2002). Recently, it was shown that lymphomas in p53-deficient mice exhibit the frequent deletion of the Aurora A gene and/or reduced protein expression, whereas normal tissue from the same mutant mice had increased Aurora A protein levels (Mao et al., 2007). These apparent discrepancies between flies and mammalian cells are currently unresolved, and elucidating the function, if any, of Aurora A and Polo during mammalian neurogenesis will be of great interest.
Cyclin E can act downstream of homeotic genes to convert a symmetric division into an asymmetric division
Cyclin E, a G1/S cyclin, is a molecule with a key role in regulating the G1- to S-phase transition. It also plays a necessary and sufficient role in making the thoracic neuroblast 6-4 (NB6-4t) divide asymmetrically, whereas its abdominal counterpart (NB6-4a) does not (Berger et al., 2005). NB6-4t localizes Prospero asymmetrically and divides to produce a Prospero+ glioblast daughter and a Prospero− neuroblast daughter (which produces only neurons). In contrast, NB6-4a divides symmetrically to produce two Prospero+ daughters of glial fate. This thoracic versus abdominal difference appears to be imposed by the differential expression of cyclin E in NB6-4t but not NB6-4a. In cyclin E mutants, both NB6-4t and NB6-4a fail to localize Prospero, and both divide symmetrically to produce daughters of glial fate. Conversely, the ectopic expression of cyclin E in NB6-4a is sufficient to cause it to divide asymmetrically like NB6-4t. Cyclin E expression is negatively regulated by genes of the bithorax complex; thus, in NB6-4a, in the abdominal neuromeres where AbdA and AbdB are expressed, cyclin E expression is repressed. The role of cyclin E in mediating asymmetric division and specifying cell fate appears to be independent of its role in cell proliferation. Neither loss nor gain of function of Decapo, the Drosophila homologue of the P21/Cip/Kip family of cyclin E–Cdk complex inhibitors, or dE2F, which is activated by cyclin E and required for the initiation of S phase, had any effect on cell fate in the NB6-4a or NB6-4t lineages, although cell numbers were affected. Thus, cyclin E can apparently act independently of its role in proliferation and downstream of homeotic function to autonomously specify the NB6-4t asymmetric division.
Cyclin E has also been attributed to have the ability to confer self-renewing asymmetric division potential to GMCs (Bhat and Apsel, 2004) and the establishment of cortical polarity in Caenorhabditis elegans (Cowan and Hyman, 2006). Cyclin E expression has been reported to be down-regulated by the fate-determining factor Tramtrack in the asymmetric divisions of the Drosophila sensory bristle lineage (Audibert et al., 2005). Up-regulation of cyclin E has been observed in both imaginal and brain tumors (Moberg et al., 2001; Betschinger et al., 2006; Wang et al., 2006). Interestingly, elevated levels of cyclin E have also been observed in a subset of human tumors, including those of the breast and ovary (Keyomarsi and Herliczek, 1997).
Anaphase-promoting complex/cyclosome function is required for the asymmetric localization of Miranda and its cargo proteins
The anaphase-promoting complex/cyclosome (APC/C) is a protein complex with at least 11 core subunits that functions as an E3 ubiquitin ligase that normally targets proteins for degradation via the 26S proteasome (Peters, 2006). Transient associations with the activating subunits Cdc20 and Cdh1 promote mitotic transitions via several key processes, including the destruction of mitotic cyclins and inhibitors of chromosome separation as well as the regulation of DNA replication, centrosome duplication, and mitotic spindle assembly (Sigrist et al., 1995; Zur and Brandeis, 2001; Leismann and Lehner, 2003). APC/C activity has recently been shown to have cell cycle–independent roles, including the control of axon growth and patterning in the developing mammalian brain (Konishi et al., 2004), the regulation of synaptic size and transmission in both C. elegans and Drosophila (Juo and Kaplan, 2004; van Roessel et al., 2004), and establishing the anterior–posterior axis of the C. elegans zygote by asymmetrically distributing Par proteins and promoting association of the paternal pronucleus/centrosome with the actin-rich cortex (Rappleye et al., 2002).
Recent findings suggest that APC/C core function is specifically required for asymmetric localization of Miranda and its interacting proteins Prospero, Brat, and Staufen but for none of the other asymmetrically localized components of the Drosophila neuroblast asymmetry machinery (Slack et al., 2007). Mutations in any one of several APC/C core components cause Miranda and its associated proteins to mislocalize to a pericentrosomal region, the nature of which is currently undefined. Mislocalization to this compartment requires neither intact microtubules nor intact centrosomal function. Although typical APC/C mutants are arrested at metaphase with high Cdk1 activity, the delocalization of Miranda appears to be largely independent of these defects. Miranda can be ubiquitinated both in vivo and in S2 cells. The extreme C-terminal region of Miranda contains a putative APC/C motif, and removal/replacement of this region prevents Miranda ubiquitination in S2 cells. Correlating with this disruption to ubiquitination, the mutant Miranda mislocalizes to the pericentrosomal compartment in a microtubule-independent manner. Interestingly, replacement of this C-terminal region with a ubiquitin moiety can restore asymmetric localization in dividing larval neuroblasts. Thus, APC/C seems to facilitate the ubiquitination of Miranda, which appears to be required for the asymmetric cortical localization of Miranda. Given the known function of APC/C in ubiquitin-mediated degradation, it will be interesting to determine whether Miranda is a direct substrate for APC/C.
Concluding remarks
There is increasing evidence that cell cycle regulators can impinge on the neuroblast asymmetry machinery and control various aspects of asymmetric division, including the decision of self-renewal versus differentiation. These cell cycle regulators include protein kinases, Cdc2/Cdk1, Aurora A, and Polo as well as APC core components and cyclin E. Interestingly, the basal protein component Pon has been shown to be a phosphorylation substrate of Polo kinase, providing a direct molecular link between a cell cycle regulator and a component of the asymmetry machinery. It has been shown that Cdc2/cyclin E and APC function are important for the establishment of cell polarity in the C. elegans zygote, suggesting that this regulation may be evolutionally conserved. The most intriguing observation is that some of the cell cycle regulators, including Aurora A and Polo, possess tumor suppressor activity in the Drosophila larval brain, at least in part through regulating Numb asymmetry. Currently, many questions remain. What are the additional downstream factors that are controlled by the Cdk1/Aurora A/Polo kinases in the regulation of asymmetric protein localization and progenitor self-renewal? What, if any, interplay is there between the Numb–Notch pathway on the one hand and Brat–Prospero on the other in regulating neuroblast self-renewal? How general a role will ubiquitination play in the process of asymmetric protein localization and asymmetric division? Future studies will provide insight into these issues.
Acknowledgments
We thank the reviewers for helpful comments.
Financial support was received from the Temasek Life Sciences Laboratory.
Abbreviations used in this paper: APC/C, anaphase-promoting complex/cyclosome; aPKC, atypical PKC; GMC, ganglion mother cell.
References
- Almeida, M.S., and S.J. Bray. 2005. Regulation of post-embryonic neuroblasts by Drosophila Grainyhead. Mech. Dev. 122:1282–1293. [DOI] [PubMed] [Google Scholar]
- Ando, K., T. Ozaki, H. Yamamoto, K. Furuya, M. Hosoda, S. Hayashi, M. Fukuzawa, and A. Nakagawara. 2004. Polo-like kinase 1 (Plk1) inhibits p53 function by physical interaction and phosphorylation. J. Biol. Chem. 279:25549–25561. [DOI] [PubMed] [Google Scholar]
- Audibert, A., F. Simon, and M. Gho. 2005. Cell cycle diversity involves differential regulation of Cyclin E activity in the Drosophila bristle cell lineage. Development. 132:2287–2297. [DOI] [PubMed] [Google Scholar]
- Beaucher, M., E. Hersperger, A. Page-McCaw, and A. Shearn. 2007. Metastatic ability of Drosophila tumors depends on MMP activity. Dev. Biol. 303:625–634. [DOI] [PubMed] [Google Scholar]
- Bello, B., H. Reichert, and F. Hirth. 2006. The brain tumor gene negatively regulates neural progenitor cell proliferation in the larval central brain of Drosophila. Development. 133:2639–2648. [DOI] [PubMed] [Google Scholar]
- Berger, C., S.K. Pallavi, M. Prasad, L.S. Shashidhara, and G.M. Technau. 2005. A critical role for cyclin E in cell fate determination in the central nervous system of Drosophila melanogaster. Nat. Cell Biol. 7:56–62. [DOI] [PubMed] [Google Scholar]
- Betschinger, J., K. Mechtler, and J.A. Knoblich. 2006. Asymmetric segregation of the tumor suppressor brat regulates self-renewal in Drosophila neural stem cells. Cell. 124:1241–1253. [DOI] [PubMed] [Google Scholar]
- Bhat, K.M., and N. Apsel. 2004. Upregulation of Mitimere and Nubbin acts through cyclin E to confer self-renewing asymmetric division potential to neural precursor cells. Development. 131:1123–1134. [DOI] [PubMed] [Google Scholar]
- Bond, J., E. Roberts, G.H. Mochida, D.J. Hampshire, S. Scott, J.M. Askham, K. Springell, M. Mahadevan, Y.J. Crow, A.F. Markham, et al. 2002. ASPM is a major determinant of cerebral cortical size. Nat. Genet. 32:316–320. [DOI] [PubMed] [Google Scholar]
- Bowman, S.K., R.A. Neumuller, M. Novatchkova, Q. Du, and J.A. Knoblich. 2006. The Drosophila NuMA homolog Mud regulates spindle orientation in asymmetric cell division. Dev. Cell. 10:731–742. [DOI] [PubMed] [Google Scholar]
- Carmena, M., M.G. Riparbelli, G. Minestrini, A.M. Tavares, R. Adams, G. Callaini, and D.M. Glover. 1998. Drosophila polo kinase is required for cytokinesis. J. Cell Biol. 143:659–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caussinus, E., and C. Gonzalez. 2005. Induction of tumor growth by altered stem-cell asymmetric division in Drosophila melanogaster. Nat. Genet. 37:1125–1129. [DOI] [PubMed] [Google Scholar]
- Choksi, S.P., T.D. Southall, T. Bossing, K. Edoff, E. de Wit, B.E. Fischer, B. van Steensel, G. Micklem, and A.H. Brand. 2006. Prospero acts as a binary switch between self-renewal and differentiation in Drosophila neural stem cells. Dev. Cell. 11:775–789. [DOI] [PubMed] [Google Scholar]
- Cowan, C.R., and A.A. Hyman. 2006. Cyclin E-Cdk2 temporally regulates centrosome assembly and establishment of polarity in Caenorhabditis elegans embryos. Nat. Cell Biol. 8:1441–1447. [DOI] [PubMed] [Google Scholar]
- Egger, B., J.M. Chell, and A.H. Brand. 2008. Insights into neural stem cell biology from flies. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363:39–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover, D.M., M.H. Leibowitz, D.A. McLean, and H. Parry. 1995. Mutations in aurora prevent centrosome separation leading to the formation of monopolar spindles. Cell. 81:95–105. [DOI] [PubMed] [Google Scholar]
- Gonzalez, C. 2007. Spindle orientation, asymmetric division and tumour suppression in Drosophila stem cells. Nat. Rev. Genet. 8:462–472. [DOI] [PubMed] [Google Scholar]
- Izumi, Y., N. Ohta, K. Hisata, T. Raabe, and F. Matsuzaki. 2006. Drosophila Pins-binding protein Mud regulates spindle-polarity coupling and centrosome organization. Nat. Cell Biol. 8:586–593. [DOI] [PubMed] [Google Scholar]
- Juo, P., and J.M. Kaplan. 2004. The anaphase-promoting complex regulates the abundance of GLR-1 glutamate receptors in the ventral nerve cord of C. elegans. Curr. Biol. 14:2057–2062. [DOI] [PubMed] [Google Scholar]
- Katayama, H., K. Sasai, H. Kawai, Z.M. Yuan, J. Bondaruk, F. Suzuki, S. Fujii, R.B. Arlinghaus, B.A. Czerniak, and S. Sen. 2004. Phosphorylation by aurora kinase A induces Mdm2-mediated destabilization and inhibition of p53. Nat. Genet. 36:55–62. [DOI] [PubMed] [Google Scholar]
- Keyomarsi, K., and T.W. Herliczek. 1997. The role of cyclin E in cell proliferation, development and cancer. Prog. Cell Cycle Res. 3:171–191. [DOI] [PubMed] [Google Scholar]
- Konishi, Y., J. Stegmuller, T. Matsuda, S. Bonni, and A. Bonni. 2004. Cdh1-APC controls axonal growth and patterning in the mammalian brain. Science. 303:1026–1030. [DOI] [PubMed] [Google Scholar]
- Lee, C.Y., R.O. Andersen, C. Cabernard, L. Manning, K.D. Tran, M.J. Lanskey, A. Bashirullah, and C.Q. Doe. 2006. a. Drosophila Aurora-A kinase inhibits neuroblast self-renewal by regulating aPKC/Numb cortical polarity and spindle orientation. Genes Dev. 20:3464–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, C.Y., K.J. Robinson, and C.Q. Doe. 2006. b. Lgl, Pins and aPKC regulate neuroblast self-renewal versus differentiation. Nature. 439:594–598. [DOI] [PubMed] [Google Scholar]
- Lee, C.Y., B.D. Wilkinson, S.E. Siegrist, R.P. Wharton, and C.Q. Doe. 2006. c. Brat is a miranda cargo protein that promotes neuronal differentiation and inhibits neuroblast self-renewal. Dev. Cell. 10:441–449. [DOI] [PubMed] [Google Scholar]
- Leismann, O., and C.F. Lehner. 2003. Drosophila securin destruction involves a D-box and a KEN-box and promotes anaphase in parallel with Cyclin A degradation. J. Cell Sci. 116:2453–2460. [DOI] [PubMed] [Google Scholar]
- Llamazares, S., A. Moreira, A. Tavares, C. Girdham, B.A. Spruce, C. Gonzalez, R.E. Karess, D.M. Glover, and C.E. Sunkel. 1991. polo encodes a protein kinase homolog required for mitosis in Drosophila. Genes Dev. 5:2153–2165. [DOI] [PubMed] [Google Scholar]
- Mao, J.H., D. Wu, J. Perez-Losada, T. Jiang, Q. Li, R.M. Neve, J.W. Gray, W.W. Cai, and A. Balmain. 2007. Crosstalk between Aurora-A and p53: frequent deletion or downregulation of Aurora-A in tumors from p53 null mice. Cancer Cell. 11:161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraldi, P., R. Honda, and E.A. Nigg. 2002. Aurora-A overexpression reveals tetraploidization as a major route to centrosome amplification in p53−/− cells. EMBO J. 21:483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberg, K.H., D.W. Bell, D.C. Wahrer, D.A. Haber, and I.K. Hariharan. 2001. Archipelago regulates Cyclin E levels in Drosophila and is mutated in human cancer cell lines. Nature. 413:311–316. [DOI] [PubMed] [Google Scholar]
- Ohshiro, T., T. Yagami, C. Zhang, and F. Matsuzaki. 2000. Role of cortical tumour-suppressor proteins in asymmetric division of Drosophila neuroblast. Nature. 408:593–596. [DOI] [PubMed] [Google Scholar]
- Peng, C.Y., L. Manning, R. Albertson, and C.Q. Doe. 2000. The tumour-suppressor genes lgl and dlg regulate basal protein targeting in Drosophila neuroblasts. Nature. 408:596–600. [DOI] [PubMed] [Google Scholar]
- Peters, J.M. 2006. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat. Rev. Mol. Cell Biol. 7:644–656. [DOI] [PubMed] [Google Scholar]
- Rappleye, C.A., A. Tagawa, R. Lyczak, B. Bowerman, and R.V. Aroian. 2002. The anaphase-promoting complex and separin are required for embryonic anterior-posterior axis formation. Dev. Cell. 2:195–206. [DOI] [PubMed] [Google Scholar]
- Sigrist, S., H. Jacobs, R. Stratmann, and C.F. Lehner. 1995. Exit from mitosis is regulated by Drosophila fizzy and the sequential destruction of cyclins A, B and B3. EMBO J. 14:4827–4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siller, K.H., C. Cabernard, and C.Q. Doe. 2006. The NuMA-related Mud protein binds Pins and regulates spindle orientation in Drosophila neuroblasts. Nat. Cell Biol. 8:594–600. [DOI] [PubMed] [Google Scholar]
- Slack, C., P.M. Overton, R.I. Tuxworth, and W. Chia. 2007. Asymmetric localisation of Miranda and its cargo proteins during neuroblast division requires the anaphase-promoting complex/cyclosome. Development. 134:3781–3787. [DOI] [PubMed] [Google Scholar]
- Tio, M., G. Udolph, X. Yang, and W. Chia. 2001. cdc2 links the Drosophila cell cycle and asymmetric division machineries. Nature. 409:1063–1067. [DOI] [PubMed] [Google Scholar]
- van Roessel, P., D.A. Elliott, I.M. Robinson, A. Prokop, and A.H. Brand. 2004. Independent regulation of synaptic size and activity by the anaphase-promoting complex. Cell. 119:707–718. [DOI] [PubMed] [Google Scholar]
- Wang, H., G.W. Somers, A. Bashirullah, U. Heberlein, F. Yu, and W. Chia. 2006. Aurora-A acts as a tumor suppressor and regulates self-renewal of Drosophila neuroblasts. Genes Dev. 20:3453–3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H., Y. Ouyang, W.G. Somers, W. Chia, and B. Lu. 2007. Polo inhibits progenitor self-renewal and regulates Numb asymmetry by phosphorylating Pon. Nature. 449:96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, F., C.T. Kuo, and Y.N. Jan. 2006. Drosophila neuroblast asymmetric cell division: recent advances and implications for stem cell biology. Neuron. 51:13–20. [DOI] [PubMed] [Google Scholar]
- Zhou, H., J. Kuang, L. Zhong, W.L. Kuo, J.W. Gray, A. Sahin, B.R. Brinkley, and S. Sen. 1998. Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nat. Genet. 20:189–193. [DOI] [PubMed] [Google Scholar]
- Zigman, M., M. Cayouette, C. Charalambous, A. Schleiffer, O. Hoeller, D. Dunican, C.R. McCudden, N. Firnberg, B.A. Barres, D.P. Siderovski, and J.A. Knoblich. 2005. Mammalian inscuteable regulates spindle orientation and cell fate in the developing retina. Neuron. 48:539–545. [DOI] [PubMed] [Google Scholar]
- Zur, A., and M. Brandeis. 2001. Securin degradation is mediated by fzy and fzr, and is required for complete chromatid separation but not for cytokinesis. EMBO J. 20:792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]