Figure 10.
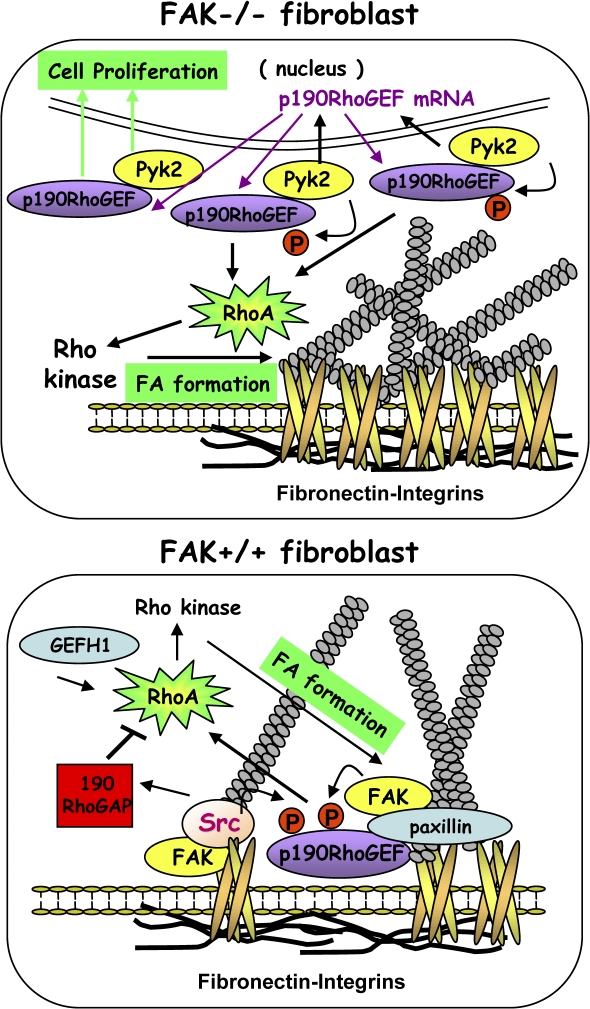
Model of p190RhoGEF function in FAK−/− and FAK+/+ MEFs. Elevated Pyk2 levels and signaling in FAK−/− MEFs promote p190RhoGEF mRNA and protein expression. Knockdown of Pyk2 reduces FAK−/− proliferation, whereas Pyk2 reexpression enhances cell growth that is dependent on p190RhoGEF expression. Pyk2 phosphorylates p190RhoGEF, Pyk2 forms a complex with p190RhoGEF that is cytoplasmic distributed, and Pyk2-p190RhoGEF acts to promote aberrant RhoA activation connected to enhanced FAK−/− MEF FA formation. Knockdown of Pyk2 or p190RhoGEF in FAK−/− MEFs restores normal integrin-regulated RhoA activity and FA formation but do not rescue FAK−/− MEF motility defects that involve lack of FA release. In normal MEFs, transient RhoA inhibition after FN plating is mediated in part through Src phosphorylation and activation of p190RhoGAP (Arthur et al., 2000). p190RhoGEF knockdown limits RhoA activation and FA formation upon FN plating, whereas p190RhoGEF overexpression overrides p190RhoGAP-mediated RhoA regulation to promote RhoA activation and enhanced FA formation and recapitulates a partial FAK−/− cell phenotype. Both p190RhoGEF overexpression and knockdown prevent cell movement as the formation of too many or too few FAs limits motility. p190RhoGEF transiently localizes to FAs upon FN replating. This is dependent on FAK binding and is associated with p190RhoGEF tyrosine phosphorylation. Unlike other cytoplasmic GEFs, such as GEFH1, that can activate RhoA, the ability of p190RhoGEF to stimulate RhoA is associated with FAK-Pyk2 binding, tyrosine phosphorylation, or FA localization. This paper has elucidated a novel role for p190RhoGEF as an integrin-proximal regulator of FA formation and cell motility.