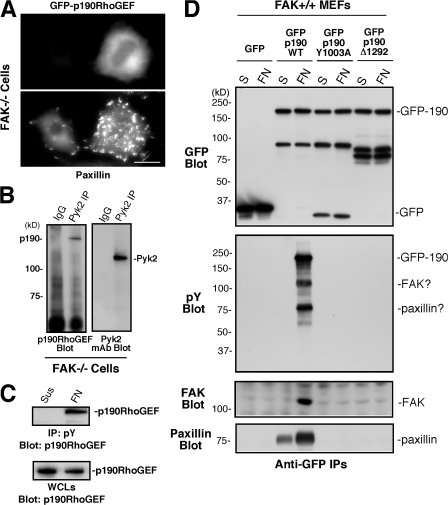
Figure 9.
Adhesion-dependent regulation of p190RhoGEF tyrosine phosphorylation. (A) GFP-p190RhoGEF is cytoplasmic distributed in FAK−/− MEFs. Cells were transiently transfected with GFP–p190RhoGEF WT, plated on FN for 1 h, and fixed and coanalyzed for GFP distribution and paxillin staining as an FA marker. Bar, 30 μm. (B) p190RhoGEF associates with Pyk2. Control rabbit IgG or anti-Pyk2 immunoprecipitations from FAK−/− MEFs were sequentially analyzed by p190RhoGEF and Pyk2 blotting. (C) p190RhoGEF is tyrosine phosphorylated upon FAK−/− MEF adhesion to FN. Antiphosphotyrosine (pY) immunoprecipitates were made from lysates of FAK−/− MEFs held in suspension or FN replated for 1 h and were analyzed by anti-p190RhoGEF blotting. The amount of p190RhoGEF in suspended or FN-replated whole cell lysates (WCLs) was determined by blotting. (D) The FAK-binding region is important for FN-stimulated p190RhoGEF tyrosine phosphorylation. Lysates were prepared from FAK+/+ MEFs expressing GFP or the indicated GFP-p190RhoGEF constructs after cell suspension or FN replating for 1 h, and anti-GFP immunoprecipitates were sequentially blotted for phosphotyrosine and GFP. In a second experiment, anti-GFP immunoprecipitates were sequentially blotted for FAK and paxillin.