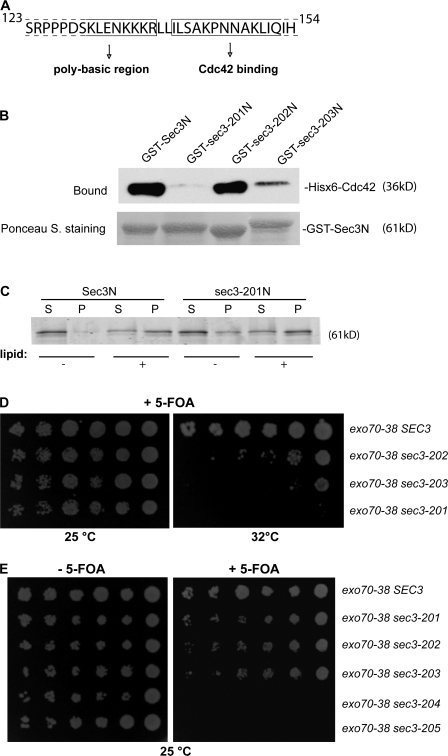
Figure 6.
The Cdc42 binding domain and the polybasic region of Sec3 are important for Sec3 function. (A) Diagram of the Sec3 sequence containing the potential Cdc42 binding site and the polybasic region. Residues before R137 may be involved in lipid binding and residues after I140 may be involved in Cdc42 binding. (B) The sec3-201 mutant was not able to bind to Cdc42 in vitro. GST fusion proteins containing the N terminus (aa 1–320) of Sec3, sec3-201 (I140A, L141A, S142A, and P145A), sec3-202 (K134A, K135A, K136A, and R137A), and sec3-203 (K134E, K135E, K136E, and R137E) were purified and conjugated to glutathione sepharose. Cdc42 was expressed as a Hisx6 fusion and purified from bacteria. The in vitro binding assay was performed using GST-Sec3N and Hisx6-Cdc42 in the presence of GTPγS. The Hisx6-Cdc42 fusion protein bound to the GST-Sec3N sepharose was detected by Western blotting with anti-Hisx6 antibody (top). Equal amounts of wild-type and mutant Sec3 fusion proteins were used in the binding assay (bottom; Ponceau S staining). Cdc42 bound to GST-Sec3N and GST–sec3-202N but not to GST–sec3-201N. GST–sec3-203N has reduced binding to Cdc42. (C) Mutations at the Cdc42 binding domain do not impair Sec3 binding to phospholipids. GST fusion proteins of wild-type Sec3N and sec3-201N mutant were incubated with 30 μM LUV containing 2% PIP2 and 20% PS. Proteins in supernatant and pellet were analyzed by SDS-PAGE. + and −, with or without phospholipids in the protein-lipid binding assays, respectively. (D) Synthetic growth defects of sec3 mutants with exo70-38. sec3-201 has mutations within the Cdc42 binding domain. sec3-202 and sec3-203 have mutations at the polybasic region (K/R→A or K/R→E, respectively). exo70-38, exo70-38 sec3-201, exo70-38 sec3-202, and exo70-38 sec3-203 were serially diluted and spotted onto SC medium plates. Cells were incubated at 25 or 32°C for 5 d. All sec3 mutants showed clear synthetic growth defects with exo70-38 at 32°C, whereas sec3-201 had the greatest defects and was unable to survive with exo70-38 over 32°C. (E) sec3 mutants with combined mutations at both the Cdc42 binding domain and polybasic region are synthetic lethal with exo70-38. Various sec3 mutants expressed under the SEC3 promoter in CEN plasmids were introduced into exo70-38 supplemented with a CEN, URA3, SEC3 balancer. sec3-204 has combined mutations of sec3-201 and sec3-202. sec3-205 combines the mutations of sec3-201 and sec3-203. The cells were serially diluted onto SC plates with or without 5-FOA and incubated for 5 d at 25°C. The sec3-204 and sec3-205 mutants were synthetic lethal with exo70-38 at 25°C.