Abstract
Combination therapies aim to overcome the limitations of individual drugs by selecting diverse targets of action to enhance effectiveness synergistically. This article reviews the principles of combination therapy and its applications for benign prostatic hyperplasia and overactive bladder. It then examines pathophysiological, pharmacological, and clinical evidence for currently available drug and device combinations for erectile dysfunction that has not responded to first-line, single-agent therapy.
Key words: Benign prostatic hyperplasia, Combination therapy, Erectile dysfunction, Intracavernosal therapy, Intraurethral therapy, Overactive bladder
Combination therapies for disease processes have evolved with increasing knowledge of disease causation and mechanisms of drug actions. It is recognized that the traditional concept of single disease, sole cause is no longer valid, and that the effect of therapy varies among individuals with the same disease process. Combination therapies aim to overcome individual drug limitations, which may include lack of effectiveness, progressive decline in effectiveness over prolonged use, or adverse events. Most drug combinations select diverse targets of action for each of the individual drugs and aim to enhance effectiveness synergistically. Alternatively, one molecule may improve the action of another by increasing its penetration or preventing its destruction or efflux.
Principles of Combination Therapy
Development of combination strategies requires detailed understanding of the pathophysiologic processes causing a disease. Conditions where more than 1 molecular site can be therapeutically targeted are ideally suited. To be considered for combination therapy, the 2 agents must act at 2 different sites, result in efficacy greater than either agent alone, and have side effects that are not greater than the sum for the 2 agents given individually at the used doses.
Combination therapy may consist of 2 agents administered independently of each other or in a single formulation. Single formulation requires that the 2 agents have similar routes of administration, similar dosage schedules, and lack of chemical interaction that could result in degradation or alteration of either drug. The advantages of single formulation over sequential administration include increased compliance, possibly reduced cost, and less likelihood of dosage-related issues. On the other hand, single formulations preclude independent titration of the 2 agents.
Combination Therapy in Non-Urological Conditions
Combination drug therapies have become a standard of care in a number of non-urologic conditions, including cancers, asthma, and type 2 diabetes mellitus.1 These combinations are now available as single-pill formulations and increase compliance over separate pills. In oncology, combination therapies include sensitizer agents that increase the effect of DNA-damaging drugs and combinations of 5-fluorouracil, leucovorin, and oxaliplatin for colon cancer.1 In diabetic patients who fail to attain adequate glycemic control with the first-line metformin, secretagogues such as glyburide are added in single-pill combinations.1
Hypertension is a classic example of a condition that benefits from combination drug therapy. The use of 2 agents with differing modes of action results in far superior efficacy than simply increasing the dose of any single agent. The addition of hydrochlorothiazide to either bisoprolol or angiotensin-receptor blocking agents results in better pressure control at lower doses of both drugs than does high-dose monotherapy with any of the agents.2 This demonstrates the synergistic effect that is greater than the additive effect of the 2 agents. These combinations also have a significantly lower side-effect profile that approaches that of a placebo.3 It has also been demonstrated that such combinations result in higher compliance.4
Combination Therapy for Urological Conditions
Benign Prostatic Hyperplasia
Benign prostatic hyperplasia (BPH) is a common condition with histological changes present in greater than 50% of men over the age of 60 and 90% of men over the age of 85 years. Therapy is directed toward alleviating the bother and irritative symptoms commonly associated with BPH and improving quality of life. Other goals are preventing clinical progression of the disease, avoiding complications (urinary retention and renal failure), and, when indicated, functioning as an alternative to surgery.
Patients with moderate to severe lower urinary tract symptoms (LUTS), enlarged prostate size (greater than 30 cm3), and benign elevation of PSA are at risk for developing complications, and pharmacological treatment should be strongly recommended.5 The 2003 American Urological Association (AUA) guidelines for managing BPH recommended close observation (watchful waiting) in men with AUA symptoms scores of less than 7. In moderate to severe cases of LUTS (AUA symptoms scores of 8–19 and 20–35, respectively), therapeutic options should be addressed.6 Two classes of drugs provide the mainstay of pharmacological management of BPH.7 The mechanisms of action of these first-line agents differ by functioning either to relax prostate muscle tone or to reduce prostate volume.8
Alpha blockers reduce the smooth-muscle tone in the stroma and prostate capsule, resulting in acute relaxation of the prostatic tissue. Alpha blockers thus provide rapid relief of symptoms by relieving the bladder outlet obstruction.5,6 Five alpha-reductase inhibitors (5-ARIs) have also shown efficacy in management of LUTS symptoms of BPH. They competitively inhibit conversion of testosterone to its active form, dihydrotestosterone (DHT). Because DHT promotes proliferation of prostatic cells and angiogenesis and also suppresses apoptosis, 5-ARIs reduce the bulkiness of the prostate tissue, reduce vasculature, and increase apoptosis.5 These benefits may not be observed, however, until 6 months after initiation of 5-ARI therapy.7,8 There is no evidence that circulating levels of DHT increase with age in men with BPH.
The long-term effects of alpha blockers (doxazosin) and 5-ARIs (finasteride) alone and in combination were studied to measure the risk of clinical progression of BPH.10 The untoward progression to urinary retention, incontinence, renal insufficiency, and recurrent urinary tract infection was compared with therapies alone or in combination. The results concluded that doxazosin and finasteride in combination were safe, significantly reduced the risk of progression, and were superior to monotherapy of either drug alone.
The rationale for combination therapy is based largely on the observation of clinical progression with doxazosin or finasteride alone. The signs associated with clinical progression, along with a 4-point AUA symptoms score increase, are the existing parameters for reliable measurement of therapeutic benefits. Several studies have evaluated combination therapy with alpha blockers and 5-ARIs for BPH. Some trials were limited to the observed longitudinal changes in AUA symptoms score and maximal urine flow rate, but from 1993 to 1998 the Medical Therapy of Prostatic Symptoms (MTOPS) study investigated the impact of combination pharmacological therapy on reducing clinical progression of BPH and the long-term risk of complications.10
The MTOPS study found that combination therapy was superior to monotherapy at decreasing clinical progression, but it also found that finasteride alone was as effective as combination therapy at reducing the risk of acute urinary retention.10 This observation was suggested because finasteride has been recognized as an agent that shrinks prostate size. In comparison, the growth of the prostate will continue in the presence of doxazosin monotherapy until the relaxation of the prostatic tissue is overcome by progressive growth, and eventually obstruction occurs. The benefits of doxazosin and finasteride alone or in combination have been critically studied. It has been proven that long-term treatment with combination therapy is safe and effective for relieving symptoms in men with a high risk of clinical progression.6,10
Overactive Bladder
Overactive bladder (OAB), first described over 100 years ago by Dudley,11,12 affects approximately 33 million Americans.11,13 OAB is a complex grouping of symptoms that include frequency, urgency with or without urge incontinence, and nocturia in the absence of an identifiable pathology.
Frequency and urgency are the principal symptoms affecting about 70% of people with OAB.14 Approximately 37% of patients with OAB experience incontinence. Patients are compelled to seek medical attention because the symptoms affect their quality of life with limitations on physical, sexual, social, and work-related activities.15 The cause of overactive bladder is not clearly understood, though idiopathic, behavioral, anatomic, intravesical, myogenic, and neurogenic factors have been implicated. Symptoms are normally secondary to detrusor overactivity,14,16 which is characterized by involuntary contractions of the detrusor during the filling phase as observed on urodynamics. In men, detrusor overactivity may often coexist with obstructive BPH.12 In the absence of pathology, detrusor instability is considered in a bladder that has been shown to contract spontaneously or when provoked because of involuntary increase in detrusor pressure. Urodynamics may show a phasic or wave-form pattern or a hypo-compliant or linear pattern of detrusor overactivity.14
Current approaches to OAB management range from behavioral modifications to surgery.15,16 There is strong evidence that behavioral therapy is highly effective, but lack of compliance limits its long-term efficacy.15 Anticholinergics have been the mainstay of pharmacologic therapy for OAB in women and men, although some clinicians have reserved the use of anticholinergics in men only if symptoms persist after intervention to decrease prostate size.16,17
It has been suggested, based on molecular cloning, that the bladder possesses 5 different subtypes of muscarinic receptors, M1 to M5.18 M2 and M3 are considered the main subtypes responsible for contractile activity in the detrusor muscle. Numerous organs throughout the body have similar muscarinic receptors. Drugs administered for OAB bind to receptors in these organs with varying degrees of affinity and result in different side effects. This is responsible for the dry mouth seen even with therapeutic dosage of antimuscarinics for OAB. The M3 subtype, though not as abundant as M2, appears to play a very important role in mediating direct contraction of the detrusor muscle.13
Oxybutynin and tolterodine are 2 typical anticholinergic agents causing blockade of the muscarinic receptors. The Food and Drug Administration approved an extended-release formulation of oxybutynin in 1998 and long-acting tolterodine in 2000, both for treatment of overactive bladder.17 Transdermal preparations are now available; the major difference offered by this preparation is avoidance of first-pass metabolism.12
BPH and the associated bladder outlet obstruction (BOO) may exacerbate OAB symptoms. Abrams and colleagues19 suggested that myogenic and neurogenic mechanisms are involved with detrusor overactivity associated with BOO. These patients would usually have symptoms related to BOO (slow stream, hesitancy, intermittency) as well as to OAB (frequency, urgency, urge incontinence).
The AUA has recommended initiation of pharmacological treatment when the level of frustration tolerance is lowered in patients and is gauged by how the patient views the impact of the symptoms on quality of life according to the International Prostate Symptoms Score (IPSS).20 The IPSS additionally measures efficacy of treatment after a score of 12 or higher has been identified.21 Among men who desire treatment, there is a theoretical concern that antimuscarinic effect on detrusor contractility may cause voiding difficulties or urinary retention in patients with BOO. Abrams’ group studied the safety of tolterodine in men with OAB and BOO, however, and concluded that tolterodine was safe and well tolerated and did not exacerbate preexisting LUTS.19 Their results also suggested that concerns about aggravating the voiding difficulties and urinary retention by the inhibitory effects of muscarinics have been exaggerated.
A large-scale, randomized, double-blinded, placebo-controlled study investigated the effectiveness of mono and combination pharmacotherapy with alpha blockers and antimuscarinics in men with LUTS from BOO and OAB. The results demonstrated a statistically significant therapeutic improvement.16 Thus, the rationale for combination therapy is based on the physiology of alpha antiadrenergic receptors and muscarinic receptors. The combination works on 2 components of the detrusor function and would potentially be more beneficial than monotherapy.22
Combination Therapy for Erectile Dysfunction
It has been estimated that over 30 million American men are unable to attain and/or maintain penile erections sufficient for satisfactory sexual performance. The etiological factors most commonly reported with this condition are arterial insufficiency and veno-occlusive dysfunction within the setting of cardiovascular disease; psychogenic and endocrinologic factors are also implicated. The aim of therapy is to restore rigid erections in order to improve the quality of erection, the frequency of penetration, and the overall sexual experience. Current pharmacologic approaches include oral therapies (phosphodiesterase-5 [PDE-5] inhibitors), intracavernosal injections, and intraurethral applications. The selection of an agent, however, depends on the underlying etiology, disease severity, treatment success, and modality tolerance.
Physiology of Penile Erections
Penile erections depend on 3 separate components: the central nervous system, the peripheral nervous system, and the corpora cavernosa. Normal erections require a complex interplay of hormones, nerves, blood vessels, and muscles. Within the central nervous system, erections seem to be mediated by a combination of alpha2 adrenergic and dopaminergic pathways. Dopaminergic agonists such as apomorphine and yohimbine may, thus, play a role in the management of erectile dysfunction (ED). The peripheral nervous system helps transmit the central impulses to the end organ. Sympathetic pathways generally provide inhibitory impulses, whereas parasympathetic and somatic innervation is pro-erectogenic.
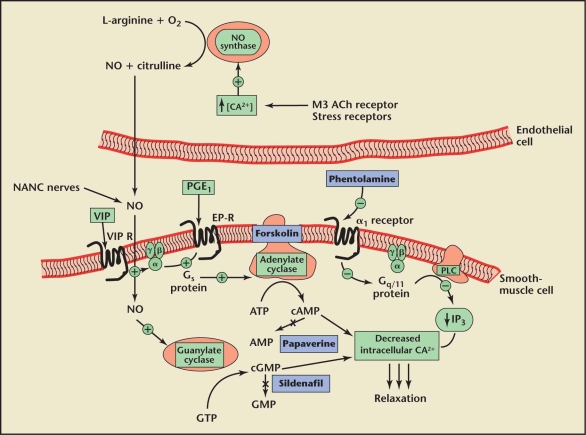
The most important component responsible for penile erections is the corpus cavernosum. Smooth-muscle relaxation within this tissue is the endpoint of all stimuli resulting in an erection. The smooth muscle has, thus, been the target for most pharmacologic therapy for ED. Figure 1 shows the molecular basis of penile erections and the sites of action of pharmacotherapeutic agents.23 The 2 main molecular pathways mediating erections are through production of cyclic guanosine monophosphate (cGMP) and cyclic adenosine monophosphate (cAMP). cGMP is produced from guanosine triphosphate (GTP) by the action of guanylate cyclase (GC). cAMP is produced from adenosine triphosphate (ATP) by the action of adenylate cyclase (AC). Both cGMP and cAMP cause a decrease in intracellular calcium levels that results in smooth-muscle relaxation.
Figure 1.
Intracellular mechanisms of various neurotransmitters, vasoactive factors, and sites of action of pharmacotherapy for erectile dysfunction. Nitric oxide (NO), synthesized via nonadrenergic, noncholinergic (NANC) nerves and endothelial cells, diffuses into the smooth-muscle cell, a process that activates guanylate cyclase and increases intracellular cyclic guanosine monophosphate (cGMP) synthesis. Sildenafil inhibits phosphodiesterase type 5 and blocks this breakdown. Prostaglandin (PGE1) and vasoactive intestinal peptide (VIP) bind to specific Gs-protein-coupled receptors, which activate adenylate cyclase and increase intracellular cyclic adenosine monophosphate (cAMP) synthesis. Forskolin directly activates adenylate cyclase. Increased cAMP and cGMP levels eventually lead to smooth-muscle relaxation. Both cAMP and cGMP are hydrolyzed to adenosine monophosphate (AMP) and guanosine monophosphate (GMP), respectively, by phosphodiesterases, terminating their effects. Papaverine non-selectively blocks both cAMP and cGMP phosphodiesterases. Alpha1-adrenergic receptors normally signal through Gq/11 heterotrimeric proteins, an outcome that activates protein kinase C-γ (PLC), liberates inositol triphosphates (IP3), and results in elevation of intracellular calcium (CA2+) and smooth-muscle contraction. Phentolamine blocks this process. ACh, acetylcholine; ATP, adenosine triphosphate; GTP, guanosine triphosphate. Reprinted from Nehra A et al23 with permission from Mayo Clinic Proceedings.
Cholinergic nerves and nonadrenergic-noncholinergic nerves mediate smooth-muscle relaxation through release of neurotransmitters such as nitric oxide (NO), vasoactive intestinal peptide (VIP), and calcitonin gene-related peptide (CGRP). NO is also released from the endothelial cells in response to increased arterial inflow. NO diffuses into the smooth-muscle cells and activates GC, resulting in an increase in cGMP. NO also causes hyperpolarization of the smooth muscle cells by activating the sodium-potassium channel. Hyperpolarization decreases intracellular calcium and increases relaxation. VIP acts through stimulation of AC and a consequent rise in intracellular cAMP levels. Prostaglandin E1 is an independent stimulator of AC through PGE1 receptors.
The production of cGMP is essential to maintain erections; ejaculation and withdrawal of sexual stimulation result in smooth-muscle contraction and detumescence by inhibiting cGMP production. cGMP can be compromised by the PDE-5 enzyme, which essentially functions as a protective mechanism by breaking down cGMP to prevent the penis from staying permanently erect. Erections require a balance between PDE-5 and cGMP; without a high level of cGMP, erections can neither be achieved nor maintained.
Existing Pharmacotherapy for ED
Currently available pharmacotherapeutic agents for ED target specific molecules in the erection pathways (Table 1). The majority of these agents act at the level of the cavernosal smooth muscles, and a few are centrally acting.23 Awareness of their site of action is critical to understanding their rational use in combination therapies.
Table 1.
Pharmacotherapeutics for Erectile Dysfunction
| Route of | ||||
|---|---|---|---|---|
| Therapeutic | Trade Name | Target | Mode of Action | Administration |
| Alprostadil | Caverject (Pharmacia, Kalamazoo, MI) | EP receptors, HCCSM | Increase cAMP, | IC, IU, |
| Edex (Schwarz Pharma, Milwaukee, WI) | SM relaxation | oral, topical | ||
| MUSE (Vivus Inc, Mountain View, CA) | ||||
| VIP + phentolamine | Invicorp* (Senetek PLC, Napa, CA) | VIP receptor agonist | Increase cAMP, | IC |
| Alpha-adrenergic | Prevents nor- | |||
| blocker | epinephrine | |||
| SM contraction | ||||
| Forskolin | - | Direct activator | Increase cAMP | IC† |
| adenylate cyclase | ||||
| Nitroglycerine | - | NO donor | Increase cGMP | Topical |
| Minoxidil | - | NO donor | Increase cGMP | Topical |
| Papaverine | - | PDE inhibitor, | Increase cAMP? | IC, topical |
| Antispasmodic | cGMP? | |||
| Milrinone | - | PDE-3 inhibitor | Increase cAMP | IC‡ |
| Sildenafil | Viagra (Pfizer Inc, New York, NY) | PDE-5 inhibitor | Increase cGMP | Oral |
| Vardenafil | Levitra (Bayer Pharmaceuticals Corp, | PDE-5 inhibitor | Increase cGMP | Oral |
| West Haven, CT) | ||||
| Tadalafil | Cialis (Lilly ICOS LLC, Indianapolis, IN) | PDE-5 inhibitor | Increase cGMP | Oral |
| Phentolamine | Regitine* (Novartis Pharmaceuticals, | Alpha-adrenergic | Prevents nor- | Oral, IC |
| Corp, East Hanover, NJ) | blocker | epinephrine | ||
| Vasomax† (Schering-Plough, | SM contraction | |||
| Kenilworth, NJ) | ||||
| Prazosin | Minipress* (Pfizer Inc, New York, NY) | Alpha-adrenergic | Prevents nor- | IC |
| blocker | epinephrine | |||
| SM contraction | ||||
| Chloropromazine | - | Alpha-adrenergic | Prevents nor- | IC |
| blocker | epinephrine | |||
| SM contraction | ||||
| Yohimbine | Yocon* (Glenwood LLC, Englewood, NJ) | Alpha2-adrenergic | CNS | Oral |
| blocker | ||||
| Trazadone | Desyrel* (Bristol-Myers Squibb Co, | Alpha2-adrenergic | CNS | Oral |
| New York, NY) | blocker | Prevents nor- | ||
| 5-HT1C agonist | epinephrine | |||
| SM contraction | ||||
| Apomorphine | Spontane* (TAP Pharmaceuticals, | Dopaminergic | CNS effects | Oral (SL) |
| Lake Forest, IL) | agonist |
Not yet approved for erectile dysfunction by the Food and Drug Administration.
Experimental use only.
Experimental use only in Germany.
cAMP, 3′5′-cyclic adenosine monophosphate; cGMP, 3′5′-cyclic guanosine monophosphate; CNS, central nervous system; HCCSM, human corpus cavernosum smooth muscle; 5-HT1c, 5-hydroxytryptamine (serotonin); IC, intracavernosal; IU, intraurethral; PDE, phosphodiesterase; SL, sublingual; SM, smooth muscle; VIP, vasoactive intestinal peptide. Modified and reprinted from Nehra A et al23 with permission from Mayo Clinic Proceedings.
cGMP-mediated action
There are 2 potential mechanisms of increasing cGMP levels within the cavernosal smooth-muscle cells. The first would increase production by increasing the available NO, and the second would decrease the breakdown of cGMP after it is produced. The latter mechanism has been instrumental in revolutionizing the treatment of ED. PDE-5 is an intracellular enzyme that catalyzes the breakdown of cGMP into GMP. This molecule can be inhibited by oral PDE-5 inhibitors such as sildenafil, tadalafil, and vardenafil. The other mechanism, that of increasing NO levels, has not been clinically successful. NO donors such as nitroglycerine, minoxidil, and linsidomine have not shown any significant effect in human trials.
cAMP-mediated action
As in cGMP mediated action, cAMP-mediated smooth-muscle relaxation can be potentiated through either an increase in the production of cAMP or a decrease in its breakdown to AMP. A number of molecules have been identified that increase cAMP production. Prostaglandin E1 (PGE1) does so by binding to specific PGE1 receptors that, coupled through G proteins, increase adenylate cyclase activity. VIP also potentiates cAMP production through specific VIP receptors. Another agent, forskolin, can augment the action of PGE1 on smooth muscles.24 Identification of specific subtypes of adenylate cyclase has enabled the development of variants of stimulators such as 6-(3-[dimethylamino] propionyl) forskolin, which has high selectivity for cardiac adenylyl cyclase and is used in the treatment of acute heart failure.25 Inhibition of cAMP breakdown can be achieved by papaverine, a nonspecific phosphodiesterase inhibitor that, to some extent, also acts on PDE-5 and increases cGMP levels.
Alpha-adrenergic inhibition
Alpha-adrenergic agents induce smooth-muscle contraction and result in detumescence. Drugs that block the action of these agents can prolong erections. These agents may be administered orally or by intracavernosal injection. Phentolamine is a non-selective alpha-adrenergic blocker. It is primarily used as an injectable agent, although some trials of oral or buccal phentolamine have also been performed. Doxazosin and yohimbine are other oral alpha-adrenergic blocking agents that have been used with limited efficacy in ED.
Other modes of action
There is little clinical relevance of agents that act through other mechanisms. These include apomorphine, a central dopaminergic agent, and endothelin receptor antagonists, which may inhibit smooth-muscle contraction within the corpus cavernosum.
Need for Combination Therapy in ED
Prior to the introduction of Viagra® (Pfizer, Inc., New York, NY) in 1998, it was estimated that only 10% of men with ED had ever used treatment, and prior to 1993 very little was known about the subject.26 The pre-Viagra armamentarium for ED treatment included penile prosthesis, vascular surgery to correct venous leakage, vacuum pump, and intracavernosal and transurethral applications of alprostadil. Today, sildenafil is recognized as the main tool for the treatment of ED.
Higher doses of sildenafil may be unsuitable in patients who report significant side effects.27 Patients who fail to respond to one PDE-5 inhibitor are very unlikely to respond to another.28 Patients who fail PDE-5 therapy are candidates for intracavernosal or intraurethral therapies and vacuum erection devices. Failure of these therapies would require a penile prosthesis. Effective combination therapies may salvage some of these failures and prevent or delay the need for prosthesis.
Combination Therapy
Intracavernosal agents
Scientifically proven therapy for ED began with the use of intracavernosal agents. Virag’s letter to the editor of Lancet regarding the use of papaverine to induce erections in patients with organic ED laid the foundation for clinical use of these agents on a routine basis.29 Papaverine, however, was used in large doses, was painful, and could lead to corporeal fibrosis. This initiated the use of combination therapies, and the combination of papaverine with alpha-adrenergic blockers and prostaglandin permitted a decrease in its dose with improved efficacy. Bechara and colleagues30 evaluated the efficacy of 40 microgram/mL prostaglandin E1 (PGE1) as single-agent therapy against a 3-drug combination of 17.64 mg/mL papaverine, 0.58 mg/mL phentolamine, and 5.8 microgram/mL PGE1 in 32 patients who had failed to respond to high doses of a 2-drug combination of papaverine (60 mg) and phentolamine (1 mg). All patients received both the 2-drug and 3-drug combination therapy in a blind, crossover fashion, at least 1 week apart. Only 22% of patients responded to PGE1, but 50% responded to the 3-drug mixture. Pain was reported by 41% of the patients receiving PGE1 monotherapy compared with 12.5% who received the 3-drug mixture. Shenfeld and colleagues31 performed a double-blind, crossover study to compare intracorporeal injections of papaverine (9 mg) plus phentolamine (0.5 mg) with a 3-drug combination of papaverine (4.5 mg), phentolamine (0.25 mg), and PGE1 (5 microgram). Twenty patients received these solutions alternately during 2 sessions. Seventy-three percent achieved full erections lasting an average of 57 minutes with the 3-drug solution compared with 28% lasting an average 33.6 minutes with the 2-drug solution.
These combinations were logically based on the differing mechanisms of action of these drugs. PGE1 activated cAMP, phentolamine inhibited the alpha-adrenoceptors, and papaverine promoted the action of the generated cAMP/cGMP by nonspecifically inhibiting phosphodiesterases.
Sildenafil and intraurethral prostaglandin
Prostaglandin and PDE-5 inhibitors may also be combined to treat oral therapy failures. This combination maintains the minimally invasive nature of therapy because the prostaglandin is placed transsurethrally and does not need to be injected. Raina and colleagues32 added the medicated urethral system for erection (MUSE® [VIVUS, Inc., Mountain View, CA]) to 23 men with post radical prostatectomy ED who were unsatisfied with sildenafil monotherapy of 100 mg. Nineteen of these 23 men (83%) reported improvement in rigidity and sexual satisfaction. Nehra and colleagues33 evaluated 28 patients, 17 post radical prostatectomy and 11 with organic ED, who had failed either sildenafil or MUSE 1000 mcg monotherapy. All patients reported improvement in their erections and were able to perform vaginal penetration with a mean of 3.6 intercourse episodes per month. Some were able to further reduce their dose of sildenafil from 100 to 50 mg.
Sildenafil and intracavernosal prostaglandin
Sildenafil may also be combined with intracavernosal prostaglandins. Mydlo and colleagues34 evaluated the combined use of intracavernosal PGE1 and oral PDE-5 inhibitors in post radical prostatectomy patients who had suboptimal response to oral therapy. Eighteen of these men had received 100 mg of sildenafil, and 16 had received 20 mg of vardenafil. These men were subsequently started on an additional 15 or 20 micrograms of intracavernosal PGE1. Twenty-two of 32 men who continued therapy reported significant improvement in erections, and some progressed to minimize the use of intracavernosal injections with sustained response.
It is also possible to alter the dosage schedule of agents when used in a combination format. Gutierrez and colleagues35 added intracavernosal PGE1 injections in a strict programmed dosage to 40 men who were dissatisfied with their oral sildenafil therapy. The patients received 4 biweekly 20 microgram intracavernous PGE1 injections along with either placebo or 50 mg of sildenafil capsules. Four weeks after initiation of therapy, the 2 groups were crossed over in terms of oral therapy. The authors found a significantly higher satisfaction rate among the group receiving PGE1 and sildenafil combination than among those receiving either sildenafil alone or PGE1-placebo combination.
Sildenafil and alpha-adrenergic antagonists
The synergistic effects of combining injectable alpha-adrenergic antagonists (phentolamine) with injectable phosphodiesterase inhibitors (papaverine) described above suggest a role for combined therapy with oral forms of both therapies or an oral with an injectable agent. Doxazosin is an oral, selective alpha1-adrenergic antagonist that acts by inhibiting the smooth-muscle tone. Kaplan and colleagues21 reported a pilot study on its use with intracavernosal therapy in men with ED who had failed prior intracavernosal therapy with alprostadil alone. Thirty-eight such men received daily doxazosin titrated to 4 mg over 3 weeks and intracavernosal therapy as needed for 12 weeks. At 12 weeks, 57.9% of patients with the combined regimen had a significant improvement in therapeutic response.
Using both oral agents, De Rose and colleagues36 enrolled 28 ED patients who had failed to respond to sildenafil alone. One group of 14 patients received sildenafil with placebo, and another received a combination of sildenafil with 4 mg of doxazosin for 30 days. Only 7.1% of patients in the placebo group showed a significant improvement in the IIEF score compared with 78.6% of patients in the combined group responding with no additional side effects.
Vacuum erection device combinations
Vacuum erection devices (VEDs) work by increasing the arterial inflow and decreasing the venous outflow from the penis. Combination of VEDs with injectable and oral agents has been reported to have a higher success rate than either agent alone. Chen and colleagues37 evaluated 10 men who had previously failed VED and injectable agent therapy for ED. They were treated with either 60 mg of papaverine or 30 micrograms of PGE1 followed by application of VED. All 10 patients responded to the combination therapy with satisfactory erections. Another study combined VED with oral sildenafil in men who were not satisfied with either agent alone.38 All 41 men who received this combination therapy reported a greater level of satisfaction than with either treatment alone.
Testosterone and sildenafil
Androgens are necessary for the modulation of NO synthesis and PDE-5 activity within the corpora cavernosa. They are also responsible for the maintenance of penile structural and functional integrity. Hypogonadism may predispose to the failure of PDE-5 inhibitor therapy in ED. Shabsigh and colleagues39 compared the efficacy of adding testosterone gel to sildenafil in patients with ED who failed to respond to sildenafil alone. All men in this multicenter trial had a morning testosterone level of 400 ng/dL or less. Patients received either a daily dose of 1% testosterone gel or placebo gel as adjunctive therapy to 100 mg sildenafil. After 12 weeks, patients receiving testosterone had greater improvement in erectile function compared with those who received placebo. A recent review by Greco and colleagues40 looked at existing data on the role of testosterone in combination with PDE-5 inhibitors and found evidence to support the use of this combination in men with ED and low or borderline normal testosterone levels.
Concerns
Most ED therapies work through reduction of smooth-muscle tone. The mechanism of action potentially can be associated with a significant drop in the systemic vascular pressure, predisposing to adverse cardiac events. There is also a potential risk of priapism, particularly in patients with primarily psychogenic impotence. Counseling regarding these 2 issues must be done in great depth with patients receiving combination therapies. Long-term data regarding safety and maintenance of efficacy with these therapies are still not available.
Conclusions
Combination therapy for ED has demonstrated significant therapeutic improvement over monotherapy. This is based on the pathophysiology of the disease, pharmacology of the drugs, and clinical evidence. Failure of first-line, single-agent therapy would be a logical indication for trial with combination therapies before progress to invasive prosthetic devices. The current knowledge of these agents is limited by the short duration of follow-up for safety and efficacy.
Main Points.
Combination therapies aim to overcome individual drug limitations, which may include lack of effectiveness, progressive decline in effectiveness over prolonged use, or adverse events.
To be considered for combination therapy, the 2 agents must act at 2 different sites, result in efficacy greater than either agent alone, and have side effects that are not greater than the sum for the 2 agents given individually at the used doses.
Combination therapy has many different applications in various disease processes including urological disease and specifically those affecting sexual function in men.
The most important component for penile erections is the corpus cavernosum. The majority of available pharmacotherapeutic agents for ED act at the level of the cavernosal smooth muscle.
The mechanism of action of most ED therapies can be associated with a significant drop in systemic vascular pressure, predisposing to adverse cardiac events. There may be a potential risk of priapism.
Although sildenafil will improve erections in most patients with ED, a significant number of patients fail sildenafil therapy, either primarily or over a prolonged period of use.
Combining prostaglandin and PDE5 inhibitors maintains the minimally invasive nature of therapy because the prostaglandin is placed transurethrally and does not need to be injected.
The combination of intracavernosal papaverine with alpha-adrenergic blockers and prostaglandin permitted a decrease in its dose with improved efficacy.
Improvement was reported by all or most of the men involved in studies that combined intracavernosal PGE1 and oral PDE5 inhibitors and vacuum erection devices with oral sildenafil, and a recent review found evidence to support the use of testosterone in combination with PDE5 inhibitors.
References
- 1.Zimmermann GR, Lehar J, Keith CT. Multi-target therapeutics: when the whole is greater than the sum of the parts. Drug Discov Today. 2007;12:34–42. doi: 10.1016/j.drudis.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Neutel JM. The role of combination therapy in the management of hypertension. Nephrol Dial Transplant. 2006;21:1469–1473. doi: 10.1093/ndt/gfk064. [DOI] [PubMed] [Google Scholar]
- 3.Frishman WH, Bryzinski BS, Coulson LR, et al. A multifactorial trial design to assess combination therapy in hypertension. Treatment with bisoprolol and hydrochlorothiazide. Arch Intern Med. 1994;154:1461–1468. [PubMed] [Google Scholar]
- 4.Mancia G, Omboni S, Grassi G. Combination treatment in hypertension: the VeraTran Study. Am J Hypertens. 1997;10(7 Pt 2):153S–158S. doi: 10.1016/s0895-7061(97)00104-0. [DOI] [PubMed] [Google Scholar]
- 5.Miner M, Rosenberg MT, Perelman MA. Treatment of lower urinary tract symptoms in benign prostatic hyperplasia and its impact on sexual function. Clin Ther. 2006;28:13–25. doi: 10.1016/j.clinthera.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Zeman PA, Siroky MB, Babayan RK. Lower urinary tract symptoms. In: Siroky M, Oates R, Babayan R, editors. Handbook of Urology: Diagnosis and Therapy. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2004. pp. 98–121. [Google Scholar]
- 7.Barkin J, Guimaraes M, Jacobi G, et al. Alpha-blocker therapy can be withdrawn in the majority of men following initial combination therapy with the dual 5alpha-reductase inhibitor dutasteride. Eur Urol. 2003;44:461–466. doi: 10.1016/s0302-2838(03)00367-1. [DOI] [PubMed] [Google Scholar]
- 8.Roehrborn CG, Schwinn DA. Alpha1-adrenergic receptors and their inhibitors in lower urinary tract symptoms and benign prostatic hyperplasia. J Urol. 2004;171:1029–1035. doi: 10.1097/01.ju.0000097026.43866.cc. [DOI] [PubMed] [Google Scholar]
- 9.Burnett AL, Wein AJ. Benign prostatic hyperplasia in primary care: what you need to know. J Urol. 2006;175(3 Pt 2):S19–S24. doi: 10.1016/S0022-5347(05)00310-1. [DOI] [PubMed] [Google Scholar]
- 10.McConnell JD, Roehrborn CG, Bautista OM, et al. Medical Therapy of Prostatic Symptoms (MTOPS) Research Group. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349:2387–2398. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- 11.Staskin DR. Overactive bladder in the elderly: a guide to pharmacological management. Drugs Aging. 2005;22:1013–1028. doi: 10.2165/00002512-200522120-00003. [DOI] [PubMed] [Google Scholar]
- 12.Garnett S, Abrams P. The natural history of the overactive bladder and detrusor overactivity. A review of the evidence regarding the long-term outcome of the overactive bladder. J Urol. 2003;169:843–848. doi: 10.1097/01.ju.0000050305.05345.40. [DOI] [PubMed] [Google Scholar]
- 13.Richelson E, Elliott DS. Advances in medical management of overactive bladder. Mayo Clin Proc. 2003;78:681–683. doi: 10.4065/78.6.681. [DOI] [PubMed] [Google Scholar]
- 14.Chapple S, MacDiarmid S. Urodynamics Made Easy. 2nd ed. Edinburgh, Scotland: Churchill Livingstone; 2000. [Google Scholar]
- 15.Wein AJ, Rackley RR. Overactive bladder: a better understanding of pathophysiology, diagnosis and management. J Urol. 2006;175(3 Pt 2):S5–S10. doi: 10.1016/S0022-5347(05)00313-7. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan SA, Roehrborn CG, Rovner ES, et al. Tolterodine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder: a randomized controlled trial. JAMA. 2006;296:2319–2328. doi: 10.1001/jama.296.19.2319. [DOI] [PubMed] [Google Scholar]
- 17.Diokno AC, Appell RA, Sand PK, et al. Prospective, randomized, double-blind study of the efficacy and tolerability of the extended-release formulations of oxybutynin and tolterodine for overactive bladder: results of the OPERA trial. Mayo Clin Proc. 2003;78:687–695. doi: 10.4065/78.6.687. [DOI] [PubMed] [Google Scholar]
- 18.Maruyama S, Oki T, Otsuka A, et al. Human muscarinic receptor binding characteristics of antimuscarinic agents to treat overactive bladder. J Urol. 2006;175:365–369. doi: 10.1016/S0022-5347(05)00017-0. [DOI] [PubMed] [Google Scholar]
- 19.Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Subcommittee of the International Continence Society. Am J Obstet Gynecol. 2002;187:116–126. doi: 10.1067/mob.2002.125704. [DOI] [PubMed] [Google Scholar]
- 20.Rosen RC. Lower urinary tract symptoms and sexual dysfunction: additional evidence of an association. BJU Int. 2004;93:689–690. doi: 10.1111/j.1464-410X.2003.04767.x. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan SA, Reis RB, Kohn IJ, et al. Combination therapy using oral alpha-blockers and intracavernosal injection in men with erectile dysfunction. Urology. 1998;52:739–743. doi: 10.1016/s0090-4295(98)00388-4. [DOI] [PubMed] [Google Scholar]
- 22.MacDonald R, Wilt TJ. Alfuzosin for treatment of lower urinary tract symptoms compatible with benign prostatic hyperplasia: a systematic review of efficacy and adverse effects. Urology. 2005;66:780–788. doi: 10.1016/j.urology.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Nehra A, Barrett DM, Moreland RB. Pharmacotherapeutic advances in the treatment of erectile dysfunction. Mayo Clin Proc. 1999;74:709–721. doi: 10.4065/74.7.709. [DOI] [PubMed] [Google Scholar]
- 24.Mulhall JP, Daller M, Traish AM, et al. Intracavernosal forskolin: role in management of vasculogenic impotence resistant to standard 3-agent pharmacotherapy. J Urol. 1997;158:1752–1758. doi: 10.1016/s0022-5347(01)64118-1. [DOI] [PubMed] [Google Scholar]
- 25.Iwatsubo K, Okumura S, Ishikawa Y. Drug therapy aimed at adenylyl cyclase to regulate cyclic nucleotide signaling. Endocr Metab Immune Disord Drug Targets. 2006;6:239–247. doi: 10.2174/187153006778249994. [DOI] [PubMed] [Google Scholar]
- 26.Katzenstein L. Viagra: The Remarkable Story of the Discovery and Launch. New York, NY: Medical Information Press; 2001. [Google Scholar]
- 27.Fagelman E, Fagelman A, Shabsigh R. Efficacy, safety, and use of sildenafil in urology practice. Urology. 2001;57:1141–1144. doi: 10.1016/s0090-4295(01)00984-0. [DOI] [PubMed] [Google Scholar]
- 28.Lau DH, Kommu S, Mumtaz FH, et al. The management of phosphodiesterase-5 (PDE5) inhibitor failure. Curr Vasc Pharmacol. 2006;4:89–93. doi: 10.2174/157016106776359871. [DOI] [PubMed] [Google Scholar]
- 29.Virag R. Intracavernous injection of papaverine for erectile failure. Letter to the Editor. Lancet. 1982;2:938. doi: 10.1016/s0140-6736(82)90910-2. [DOI] [PubMed] [Google Scholar]
- 30.Bechara A, Casabe A, Cheliz G, et al. Prostaglandin E1 versus mixture of prostaglandin E1, papaverine and phentolamine in nonresponders to high papaverine plus phentolamine doses. J Urol. 1996;155:913–914. [PubMed] [Google Scholar]
- 31.Shenfeld O, Hanani J, Shalhav A, et al. Papaverine-phentolamine and prostaglandin E1 versus papaverine-phentolamine alone for intracorporeal injection therapy: a clinical double-blind study. J Urol. 1995;154:1017–1019. [PubMed] [Google Scholar]
- 32.Raina R, Nandipati KC, Agarwal A, et al. Combination therapy: medicated urethral system for erection enhances sexual satisfaction in sildenafil citrate failure following nerve-sparing radical prostatectomy. J Androl. 2005;26:757–760. doi: 10.2164/jandrol.05035. [DOI] [PubMed] [Google Scholar]
- 33.Nehra A, Blute ML, Barrett DM, Moreland RB. Rationale for combination therapy of intraurethral prostaglandin E(1) and sildenafil in the salvage of erectile dysfunction patients desiring noninvasive therapy. Int J Impot Res. 2002;14(suppl 1):S38–S42. doi: 10.1038/sj.ijir.3900795. [DOI] [PubMed] [Google Scholar]
- 34.Mydlo JH, Viterbo R, Crispen P. Use of combined intracorporal injection and a phosphodiesterase- 5 inhibitor therapy for men with a suboptimal response to sildenafil and/or vardenafil monotherapy after radical retropubic prostatectomy. BJU Int. 2005;95:843–846. doi: 10.1111/j.1464-410X.2005.05413.x. [DOI] [PubMed] [Google Scholar]
- 35.Gutierrez P, Hernandez P, Mas M. Combining programmed intracavernous PGE1 injections and sildenafil on demand to salvage sildenafil nonresponders. Int J Impot Res. 2005;17:354–358. doi: 10.1038/sj.ijir.3901290. [DOI] [PubMed] [Google Scholar]
- 36.De Rose AF, Giglio M, Traverso P, et al. Combined oral therapy with sildenafil and doxazosin for the treatment of non-organic erectile dysfunction refractory to sildenafil monotherapy. Int J Impot Res. 2002;14:50–53. doi: 10.1038/sj.ijir.3900815. [DOI] [PubMed] [Google Scholar]
- 37.Chen J, Godschalk MF, Katz PG, Mulligan T. Combining intracavernous injection and external vacuum as treatment for erectile dysfunction. J Urol. 1995;153:1476–1477. [PubMed] [Google Scholar]
- 38.Chen J, Sofer M, Kaver I, et al. Concomitant use of sildenafil and a vacuum entrapment device for the treatment of erectile dysfunction. J Urol. 2004;171:292–295. doi: 10.1097/01.ju.0000098460.02560.fe. [DOI] [PubMed] [Google Scholar]
- 39.Shabsigh R, Kaufman JM, Steidle C, Padma-Nathan H. Randomized study of testosterone gel as adjunctive therapy to sildenafil in hypogonadal men with erectile dysfunction who do not respond to sildenafil alone. J Urol. 2004;172:658–663. doi: 10.1097/01.ju.0000132389.97804.d7. [DOI] [PubMed] [Google Scholar]
- 40.Greco EA, Spera G, Aversa A. Combining testosterone and PDE5 inhibitors in erectile dysfunction: basic rationale and clinical evidences. Eur Urol. 2006;50:940–947. doi: 10.1016/j.eururo.2006.06.049. [DOI] [PubMed] [Google Scholar]