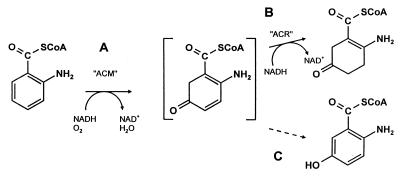
Figure 1.
Reactions catalyzed by ACMR. The monooxygenase functionality (ACM) first is reduced by NADH and subsequently reacts with dioxygen (in the presence of the substrate AB-CoA) probably to form a flavin 4a-hydroperoxide (not shown). This then inserts oxygen into AB-CoA to form the assumed 5-oxo-2-aminocyclohexadiene intermediate (A). The latter is hydrogenated rapidly by the reductase functionality (ACR) to yield the more stable AOB-CoA (B). At limiting NADH concentrations, the intermediate spontaneously rearranges to the aromatic 5-OH-AB-CoA (C). Reaction B thus constitutes an efficient trapping of the intermediate.