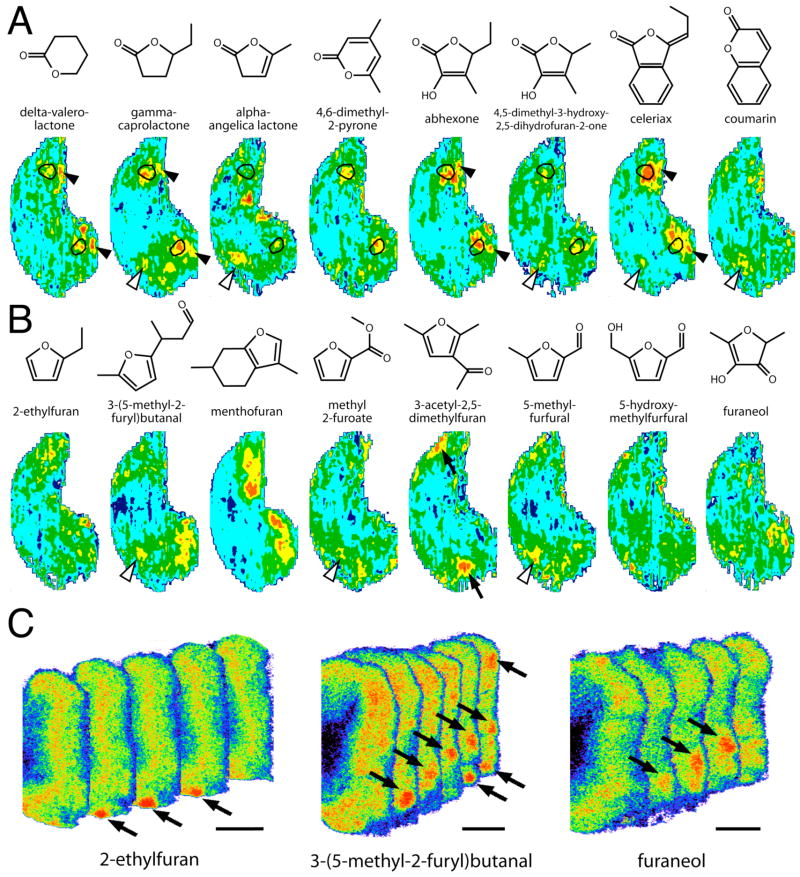
Fig. 7.
A, B: Anatomically standardized contour charts showing relative 2-DG uptake across the glomerular layer in animals exposed to heterocyclic odorants in which oxygen participates in the ring. Orientation and color scale are the same as for Figure 1. Charts represent z-score standardized data matrices averaged across different rats exposed to the same odorant condition. A: Activity patterns evoked by lactones, which are cyclic compounds having an ester bond within the ring. Most lactones activated paired regions (outlines) that we had previously identified as being responsive to open-chained esters. Some lactones also stimulated a dorsomedial area (open arrowheads) stimulated by methyl and ethyl esters that are prone to hydrolysis into corresponding carboxylic acids. Another subset of lactones stimulated very posterior, midlateral and midmedial areas (solid arrowheads) that also are stimulated by other small, hydrophilic odorants. B: Activity patterns evoked by furyl odorants. Most of these patterns were weak and apparently unrelated to each other. Certain responses appeared to be associated with functional groups present in substituents, including aldehydes (open arrowheads) and ketones (solid arrows). C: Pseudocolor-enhanced images showing stimulation of what appear to be isolated glomeruli (arrows) in the posterior, medial regions of response to each of three furyl odorants. There are 100 μm between the consecutive, 20-μm sections. Scale bars = 1 mm.