Abstract
Background/Aims. The concept of metaplastic and non-metaplastic types of gall bladder cancer and the likelihood of hormone receptor expression in the nuclei of tumour cells raised the possibility of a potential role for anti-estrogen therapy in gall bladder cancer. This study was carried out to determine the hormone receptors (ER/PR) expression level in gall bladder cancer using specific immunohistochemical assays and correlate it with patient and tumour histopathological characteristics. Patients and methods. Histopathological tumour specimens of 62 patients who underwent a radical cholecystectomy were analysed. Pronase pretreatment and primary monoclonal antibodies were used to perform immunohistochemical analysis for ER and PR. Results. The histology was adenocarcinoma – predominantly, moderately to poorly differentiated (91%). Gallstones were present in 90% of the individuals. Of the 62 specimens analysed, 62 (100%) and 61 (98%) were negative for ER and PR, respectively. Conclusion. The high incidence of gallstone-related gall bladder cancer in India is associated with metaplasia and a tendency to poorer differentiation in the tumour histology. These tumours are consequently less likely to express hormone receptors. Thus, there does not seem to be a role for anti-hormone therapy in patients with histogenesis similar to that seen in India.
Keywords: gall bladder cancer, immunohistochemistry, estrogen receptor, progesterone receptor
Introduction
Gall bladder cancer has been traditionally associated with a poor prognosis. The 5-year survival rate is <5% 1. India is the country with the highest incidence of gall bladder cancer 2.
Yamamoto et al. 3 separated gall bladder cancers into metaplastic and non-metaplastic types based on the presence or absence of metaplastic changes in the tumour tissues and their immediate vicinity. They went on to show that estrogen receptor (ER) immunoreactivity was higher in these metaplastic tumours 4. Nakamura et al. 5 found that increasing lack of differentiation (moderately and poorly differentiated lesions) was associated with more ER expression in the nuclei. He concluded that such patients could benefit from anti-hormone therapy. These initial studies, however, lacked reproducibility. This was evidenced by the work of Ko et al. 6, who showed that ER expression in tumours was absent to weak. Malik et al. 7 demonstrated that poor differentiation was more likely to be associated with negative ER expression. Further, lack of expression of Er isoform at the invasive front of the tumour was found to be associated with a more aggressive malignancy 8. It was because of these conflicting reports, the high incidence of gall bladder cancer in our country 2, and the lack of convincing and effective adjuvant treatments for gall bladder cancer, that we decided to undertake this study. We wanted to study the hormone expression in gall bladder cancer in the hope that this may provide us with data to support the use of anti-hormone as an adjunct to surgery to obtain better outcomes in these patients.
Patients and methods
Tumour specimens of 62 patients with gall bladder cancer treated at the Department of Gastrointestinal and Hepatopancreatobiliary Surgical Oncology of the Tata Memorial Hospital from 1 January 2005 to 31 January 2006 were analysed by immunohistochemical assay for the expression of ER and PR status. Data for each of the 62 patients including age, sex, liver function tests, tumour markers (CA19-9), radiological imaging, treatment carried out and follow-up (whenever possible) were strictly maintained. Every specimen was stained with haematoxylin and eosin (H&E) to obtain the diagnosis along with staging and differentiation.
Immunohistochemical assay was performed on all the postoperative histopathology specimens. Paraffin-embedded sections were dewaxed in xylene and rehydrated in a graded series of ethanol. Microwave antigen retrieval was done in citrate buffer (pH 6.0) for 10 min before peroxidase quenching with 3% H2O2 in methanol for 10 min. Primary antibodies against ER (mouse antihuman ERa clone 1D5 code: M 7047) and PR (monoclonal mouse anti-human PR clone: PgR 636 code: M 3569) were incubated for 2 h at room temperature. Biotinylated second antibody and enzyme conjugate incubation was carried out for 45 min each. Diaminobenzidine was added and incubated until chromogen developed. Specimens were evaluated by the scoring system used for breast cancer, as there is no standard scoring system in gall bladder cancer.
Results
Patient characteristics
Of the 62 patients, 18 were males and 44 were females. The mean age was 50.1±9.8 years. Fifty-six patients (90%) had associated gallstones.
Histopathological characteristics (Table I)
Table I. Tumour histopathology and grade.
| Histopathology and grade | Incidence (n=62) |
|---|---|
| Papillary adenocarcinoma | 1 (1.7%) |
| Well differentiated adenocarcinoma | 5 (8.0%) |
| Moderately differentiated adenocarcinoma | 37 (59.7%) |
| Poorly differentiated adenocarcinoma | 19 (30.6%) |
The histology was adenocarcinoma – predominantly, moderately to poorly differentiated (91%). There was one case of papillary adenocarcinoma and there were five cases of well differentiated cancer.
Hormone receptor status (Table II)
Table II. Patterns of ER and PR expression in gall bladder cancer.
| ER expression | PR expression | Number of cases |
|---|---|---|
| Negative | Negative | 57 |
| Negative | +2 (Negative) | 2 |
| Negative | +6 | 1 |
| +2 (Negative) | +1 (Negative) | 1 |
| +1 (Negative) | Negative | 1 |
| Total = 62 |
Of the 62 specimens analysed, 62 (100%) and 61 (98%) were negative for ER and PR, respectively. Two specimens had level 1+ and 2+ expression for ER (considered negative expression), while three specimens had 1+ and 2+ expression for PR. Only one specimen had 6+ expression for progesterone receptor. The only specimen positive for PR was seen in a female patient. There was no correlation with tumour stage.
Discussion
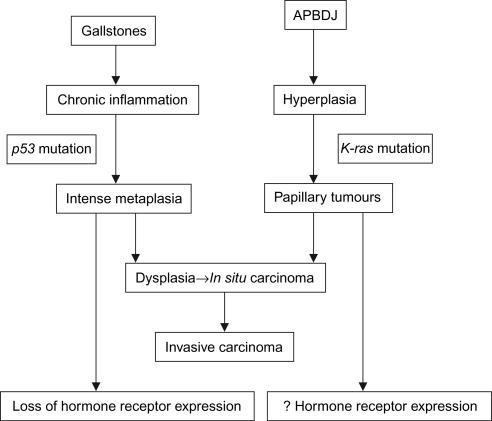
The results noted in our study, i.e. the high incidence of gallstone disease, the tendency towards moderately to poorly differentiated adenocarcinomas and the lack of expression of hormone receptors, seem to differ from the Japanese results 4,5, but are quite similar to studies from the USA and Pakistan 6,7. The study by Malik et al. 7 had 30 patients and they had even used FNAC specimens. In an endeavour to try to explain our results, disappointing as they may seem, we tried to analyse the molecular and genetic developmental history of gall bladder cancer in India and Japan. It is known that gall bladder cancer, like colorectal cancer, undergoes sequential histopathological and molecular changes from in situ to invasive carcinoma 9,10. The aetiology of gall bladder cancer has primarily been attributed to gallstones 11. In India, gallstones are quite common, especially in the North 12. However, in Japan and China, anomalous pancreaticobiliary duct junction (APBDJ) appears to be a leading cause for gall bladder cancer 12,13. The pathogenesis of gall bladder cancer as a result of the two aetiologies varies (Figure 1). In APBDJ, the inciting factor for cancer is the constant irritation of the gall bladder epithelium by the refluxing pancreatic juice and the stasis of pancreatic juice in the gall bladder 14. The result is epithelial hyperplasia associated with a high frequency of Kras mutations at codon 12 15. The resultant metaplasia is present to a much lesser degree and the resultant neoplasm is more likely to be a papillary adenocarcinoma. On the other hand, the presence of gallstones induces a severe inflammatory reaction in the mucosa and consequent metaplasia to the gastric or intestinal types 16. This ultimately leads to the development of dysplasia and carcinoma in a sequence that involves p53 mutations 10.
Figure 1. .
Algorithm showing the varying aetiologies and their progression to malignancy. APBDJ, anomalous pancreaticobiliary duct junction.
Thus, from the available data it is clear that in countries where there is a strong association of gallstones with gall bladder cancer, there are more frequent mutations of p53 and consequent metaplasia and a tendency towards poorer differentiation – a phenomenon associated with a loss of ER/PR expression. While in regions where the predominant aetiology is APBDJ and the changes follow the sequence of hyperplasia to dysplasia to in situ to invasive carcinoma along with K-ras mutations, these patients are more likely to have papillary 17 and well differentiated tumours and consequently, ER/PR expression.
In fact, Misra et al. 18 have shown a near 70% overexpression of p53 protein with a significant correlation with gallstones. A recent pilot study has also shown that p53 plays a critical role in tumour progression 19.
Based on these data, we are able to explain that the loss of hormone expression in gall bladder cancer in our study may be a feature in countries, like India, where the incidence of gallstone-induced gall bladder cancer is high. This allows limited opportunity for the role of anti-estrogen therapy.
With neoadjuvant, adjuvant, or palliative chemotherapy, and complementary radiotherapy having shown poor or ill-defined outcomes 19, at this time we believe that early detection and radical surgery continue to be the only hope for an extended survival in patients with gall bladder cancer 20.
Acknowledgements and disclosure
This study was funded by an intra-mural scientific grant from the Tata Memorial Hospital.
No disclosures.
References
- 1.National Cancer Registry Programme. Consolidated report of the population based cancer registries 1990–1996, Incidence and distribution of cancer. . New Delhi: Indian Council of Medical Research, 2001:52–3. [Google Scholar]
- 2.Perpetuo MD, Valdivieso M, Heilbrun LK, Nelson RS, Connor T, Bodey GP. Natural history study of gall bladder cancer: a review of 36 years experience at MD Anderson hospital and tumour institute. Cancer. 1978;42:330–5. doi: 10.1002/1097-0142(197807)42:1<330::aid-cncr2820420150>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto M, Nakajo S, Tahara E. Histogenesis of well differentiated adenocarcinoma of the gall bladder. Pathol Res Pract. 1989;184:279–86. doi: 10.1016/S0344-0338(89)80087-1. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto M, Nakajo S, Tahara E. Immunohistochemical analysis of estrogen receptors in human gall bladder cancer. Acta Pathol Jpn. 1990;40:14–21. doi: 10.1111/j.1440-1827.1990.tb01524.x. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura S, Muro H, Suzuki S. Estrogen and progesterone receptors in gall bladder cancer. Jpn J Surg. 1989;19:189–94. doi: 10.1007/BF02471584. [DOI] [PubMed] [Google Scholar]
- 6.Ko CY, Schmit P, Cheng L, Thompson JE. Estrogen receptors in gall bladder cancer: detection by an improved immunohistochemical assay. Am Surg. 1995;61:930–3. [PubMed] [Google Scholar]
- 7.Malik IA, Abbas Z, Shamsi Z, Daudi I, Shah HA, Moid I, et al. Immunohistochemical analysis of estrogen receptors on the malignant gall bladder tissue. J Pak Med Assoc. 1998;48:123–6. [PubMed] [Google Scholar]
- 8.Sumi K, Matsuyama S, Kitajima Y, Miyazaki K. Loss of estrogen receptor beta expression at cancer front correlates with tumor progression and poor prognosis of gall bladder cancer. Oncol Rep. 2004;12:979–84. [PubMed] [Google Scholar]
- 9.Watanabe M, Asaka M, Tanaka J, Kurosawa M, Kasai M, Miyazaki T. Point mutation of K-ras gene codon 12 in biliary tract tumours. Gastroenterology. 1994;107:1147–53. doi: 10.1016/0016-5085(94)90240-2. [DOI] [PubMed] [Google Scholar]
- 10.Wistuba II, Gazdar AF, Roa I, Albores-Saavedra J. p53 protein overexpression in gallbladder carcinoma and its precursor lesions: an immunohistochemical study. Hum Pathol. 1996;27:360–5. doi: 10.1016/s0046-8177(96)90109-4. [DOI] [PubMed] [Google Scholar]
- 11.Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer. 2006;118:1592–602. doi: 10.1002/ijc.21683. [DOI] [PubMed] [Google Scholar]
- 12.Singh V, Trikha B, Nain C, Singh K, Bose S. Epidemiology of gallstone disease in Chandigarh: a community based study. J Gastroenterol Hepatol. 2001;16:560–3. doi: 10.1046/j.1440-1746.2001.02484.x. [DOI] [PubMed] [Google Scholar]
- 13.Yagyu K, Lin Y, Obata Y, Kikuchi S, Ishibashi T, Kurosawa M, et al. JACC study group. Bowel movement frequency, medical history and the risk factor for gall bladder cancer death: a cohort study in Japan. Cancer Sci. 2004;95:674–8. doi: 10.1111/j.1349-7006.2004.tb03328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanno S, Obara T, Fujii T, Mizukami Y, Shudo R, Nishino N, et al. Proliferative potential and K-ras mutation in epithelial hyperplasia of the gall badder in patients with anomalous pancreatobiliary ductal union. Cancer. 1998;83:267–75. [PubMed] [Google Scholar]
- 15.Hanada K, Tsuchida A, Iwao T, Eguchi N, Sasaki T, Morinaka K, et al. Gene mutations of K-ras in gallbladder mucosae and gallbladder carcinoma with an anomalous junction of the pancreaticobiliary duct. Am J Gastroenterol. 1999;94:1638–42. doi: 10.1111/j.1572-0241.1999.01155.x. [DOI] [PubMed] [Google Scholar]
- 16.Roa I, De Artexabala X, Araya JC, Roa J. Preneoplastic lesions in gall bladder cancer. J Surg Oncol. 2006;93:615–23. doi: 10.1002/jso.20527. [DOI] [PubMed] [Google Scholar]
- 17.Nuzzo G, Clemente G, Caddedu F, Ardito F, Riccii R, Vecchio FM. Papillary carcinoma of the gall bladder and anomalous pancreaticobiliary junction. Report of three cases and review of literature. Hepatogastroenterology. 2005;52:1034–8. [PubMed] [Google Scholar]
- 18.Misra S, Chaturvedi A, Goel MM, Mehrotra R, Sharma ID, Srivastava AN, et al. Overexpression of p53 protein in gall bladder carcinoma in North India. Eur J Surg Oncol. 2000;26:164–7. doi: 10.1053/ejso.1999.0763. [DOI] [PubMed] [Google Scholar]
- 19.Chaube A, Tewari M, Garbyal RS, Singh U, Shukla HS. Preliminary study of p53 and c-erbB-2 expression in gall bladder cancer in Indian patients. BMC Cancer. 2006;6:126–31. doi: 10.1186/1471-2407-6-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez-Fernandez A, Gomez-Rio M, Medina-Benitez A, Moral JVD, Ramos-Font C, Ramia-Angel JM, et al. Application of modern imaging methods in diagnosis of gallbladder cancer. J Surg Oncol. 2006;93:650–64. doi: 10.1002/jso.20533. [DOI] [PubMed] [Google Scholar]