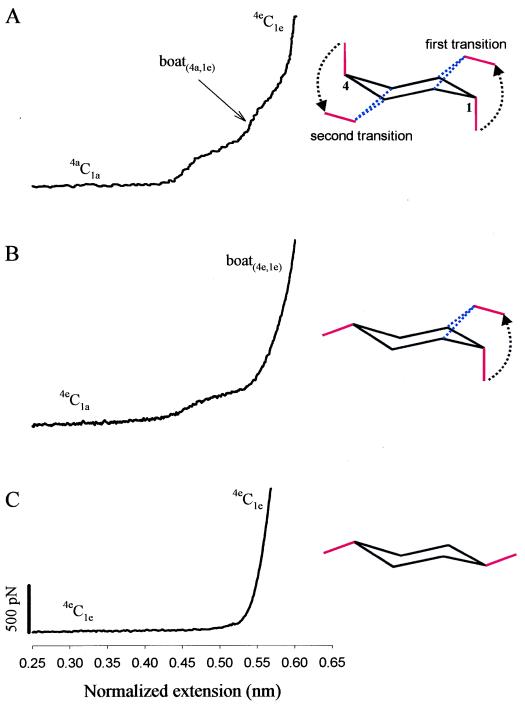
Figure 4.
A comparison of elastic characteristics of pyranose-based polysaccharides that exploit both glycosidic bonds in the axial position (galactouronan, A), one axial and one equatorial bond (amylose, B) and both equatorial bonds (methylcellulose, C). (Right) Conformational transitions that are responsible for the enthalpic extensions of the polymers. The dominant conformers of the pyranose ring are indicated along different fragments of the force extension curves. The extensions in A and B were normalized in the same way as in Fig. 2B (O1O4chair for amylose is also equal to 0.45 nm; ref. 2); in C the extension was normalized: xnorm = 0.542x/lc nm, where 0.542 nm corresponds to the length of the residue vector O1O4chair for the β-d-glucopyranose monomer of cellulose (2), and lc is the contour length of methylcellulose determined from the FJC model fit to the data in the whole force region (not shown).