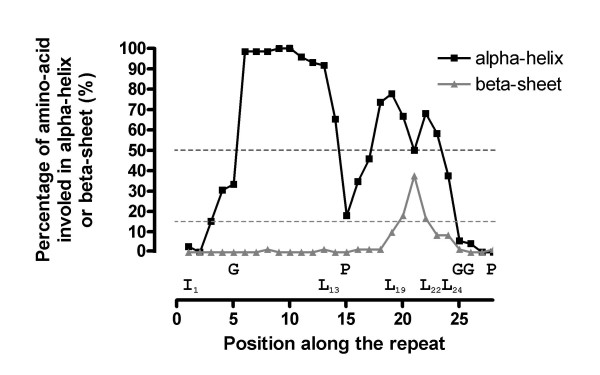
Figure 4.
Common secondary structure of the LRRs of P. amoebophila defined by the CAS approach. On each position of the consensus of the 28-residue LRRs the proportion of amino acids of the 70 LRRs of the six LGR proteins predicted by NNPREDICT to belong to either an α-helix or a β-sheet is plotted. Perturbating amino acids present in some LRR, i.e. Glycyl (G) and Prolyl (P) residues are posted as they are located on the LRR unit. This figure clearly shows that while secondary structures are present within the LRR units, no particular structural configuration could be observed at the boundaries of the units.