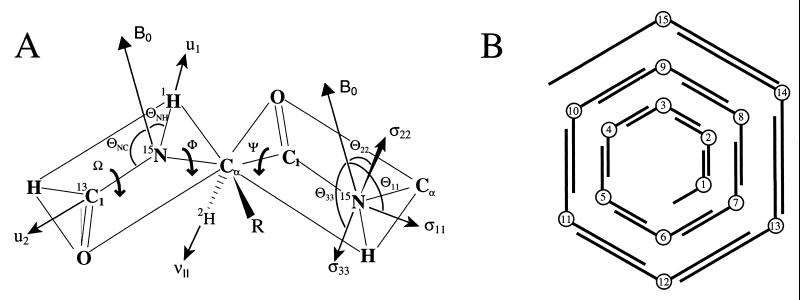
Figure 1.
(A) The primary structural constraints for the polypeptide backbone are derived from the 15N–1H and 15N–13C dipolar interactions, the Cα–2H quadrupolar interaction, and the anisotropic 15N chemical shift. The initial structure is developed by determining each peptide-plane orientation with respect to the magnetic field axis with two dipolar interactions. The relative orientations of the peptide planes is then determined (i.e., the φ and ψ angles) for a diplane structure. (B) The initial structure is assembled sequentially with overlapping diplanes. The initial structure is not a unique structure because of chirality ambiguities; however, the molecular fold, hydrogen-bonding pattern, helical sense, and residues per turn are uniquely defined.