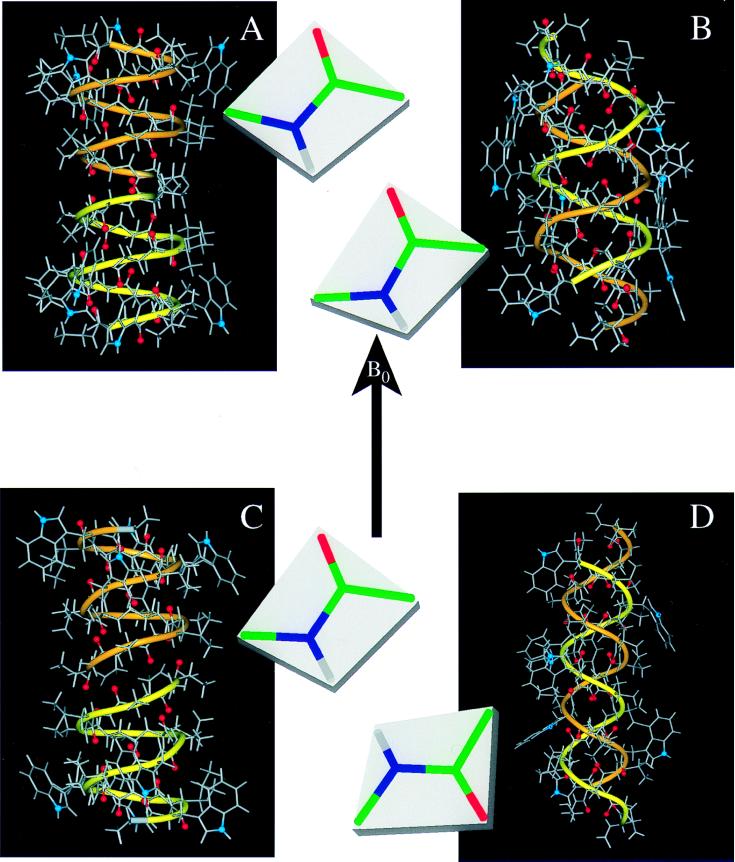
Figure 2.
Gramicidin A structures in different environments. In addition to the atomic ball-and-stick structures, a ribbon is added to accentuate the handedness and strandedness of the helix. The two monomers have different colored ribbons: one yellow and one orange. The backbone carbonyl oxygens that line the pore of the channel are highlighted in red, and the indole 15N sites, important in dictating the strandedness of the structure in a membrane environment, is shown in blue. For each structure the Ala3–Leu4 peptide-plane orientation is shown with respect to B. (A) The solid-state NMR-derived structure from a bilayer environment: single-stranded, right-handed, and 6.5 residues per turn (ref. 1; PDB accession no. 1MAG). (B) An x-ray crystallographic structure of crystals prepared from Cs+/MeOH solution: double-stranded, right-handed, and 7.2 residues per turn (ref. 5; PDB accession no. 1AV2). (C) A solution NMR structure from an SDS micellar environment: single-stranded, right-handed, and 6.3 residues per turn (ref. 4; PDB accession no. 1GRM). (D) An x-ray crystallographic structure of crystals prepared from benzene/methanol solution: double-stranded, left-handed, and 5.6 residues per turn (ref. 7; PDB accession no. 1ALZ).