Fig 2. Binding of in vitro translated β-arrestins to recombinant β2ARs reconstituted in phospholipid vesicles.
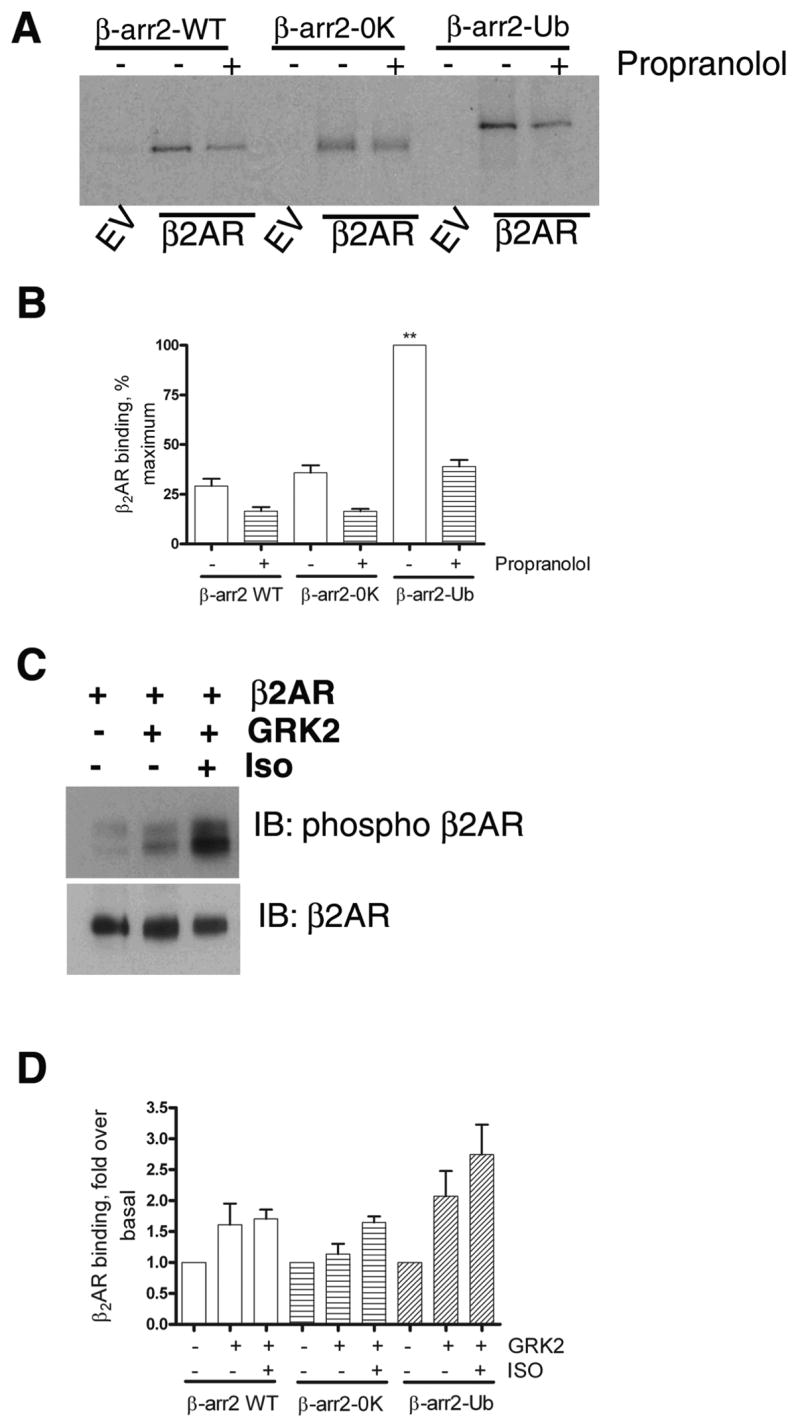
A. As described in the methods section, ‘in vitro’ translated, 35S labeled β-arrestin2, β-arrestin20K or β-arrestin2-Ub was incubated with either empty phospholipid vesicles or vesicles containing β2AR. The vesicles were then precipitated by repeated centrifugation and wash cycles. The final pellet was solubilized, separated on SDS gels and the bound β-arrestins were detected by autoradiography. In the indicated lanes, propranolol (50 μM) was included in the reaction mixture. B. Results from 3 separate binding experiments were quantified and shown as bar graphs. ** p<0.001, β-arrestin2 or β-arrestin20K versus β-arrestin2-Ub, one way ANOVA, and Tukey’s multiple comparison test. β-arrestin2 versus β-arrestin20K, no significant difference. C. Reconstituted β2AR samples were probed with a phosphoserine antibody (Serines 355, 356 within β2AR carboxyl tail) in the upper panel and with a β2AR antibody (H-20, Santacruz) in the lower panel. D. Receptor-β-arrestin binding was tested as in ‘A’. GRK2 alone or GRK2 and isoproterenol (50 μM) were added to the reaction as indicated. The bar graph is a summarization of two independent experiments performed in duplicate. For each β-arrestin form, basal binding in the absence of GRK2 and isoproterenol was assigned as 1.
