Fig 8. Isoproterenol stimulated clathrin and AP-2 binding to β-arrestin2, β-arrestin2-Ub and β-arrestin20K.
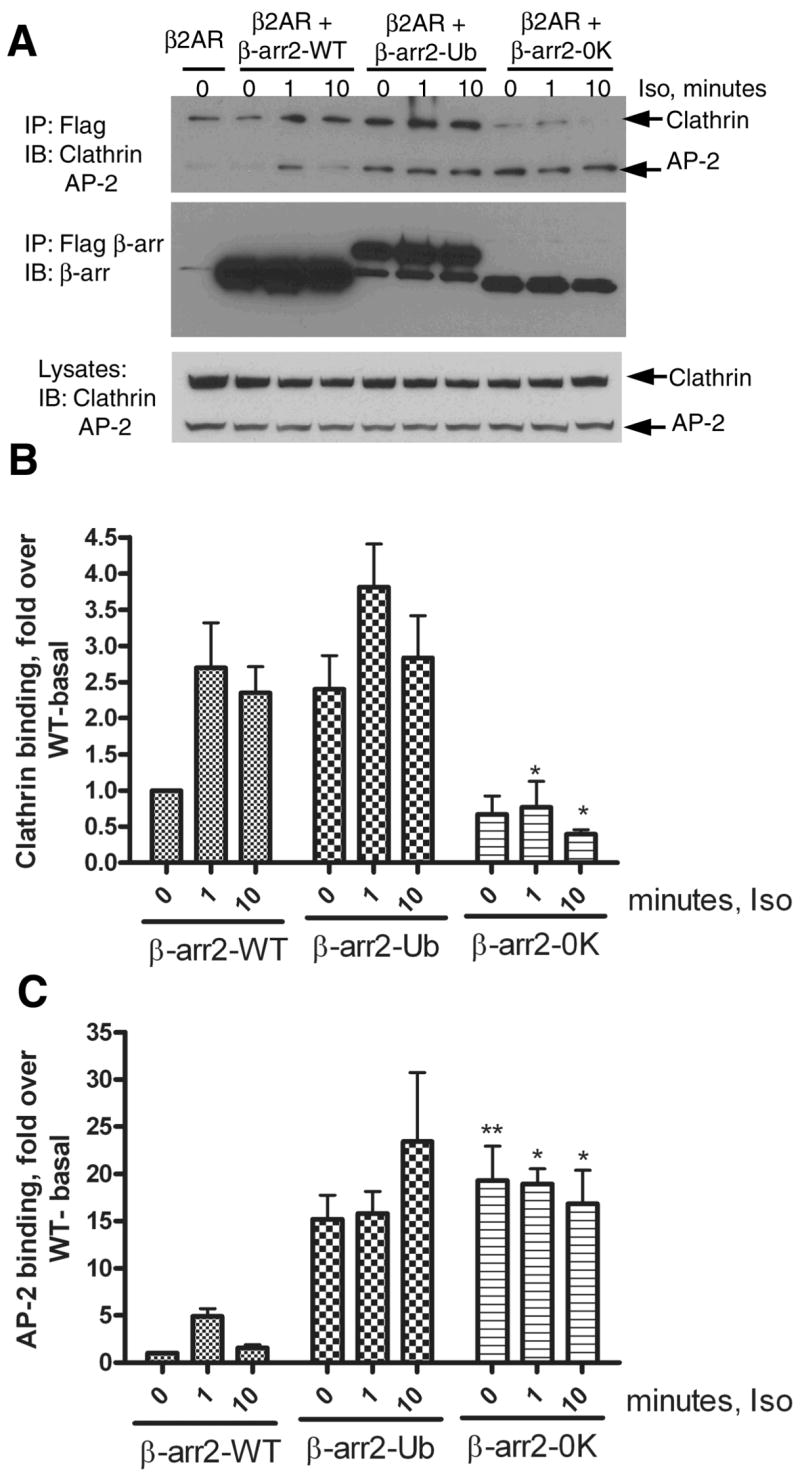
A. COS-7 cells were transiently transfected with vector or the respective FLAG-tagged β-arrestin2 plasmid and HA-β2AR. Cells were serum deprived for 1h, and stimulated for indicated times with 10 μM isoproterenol. Isolated anti-FLAG immunoprecipitates were simultaneously probed for Clathrin heavy chain and the AP-2 subunit. The bottom panel represents the endogenous levels of both these proteins in COS-7 cells as detected by the antibodies. The top panel represents the amount of Clathrin and AP-2 in β-arrestin IPs. The middle panel presents reprobing of the IP blot for β-arrestin2, β-arrestin20K and β-arrestin2-Ub with a β-arrestin antibody (A1CT). Shown are representative blots from one of four independent experiments. B. Bar graph depicts the quantification of clathrin associated with each type of β-arrestin2. Data was normalized to the amount of clathrin bound to WT under basal conditions. β-arrestin20K bound significantly lesser clathrin than the WT at 1 min and 10 min agonist treatment. *P<0.05, β-arrestin20K versus respective signal in the WT, Two way ANOVA, Bonferroni post tests. C. Bar graph shows quantification of AP-2 associated with each type of β-arrestin normalized to the levels detected in the WT immunoprecipitate under basal conditions. β-arrestin20K bound significantly higher AP2 than the WT basally and at 1 min and 10 min agonist treatment. *P<0.05, ** P<0.01 β-arrestin20K versus respective signal in the WT, Two way ANOVA, Bonferroni post tests.
