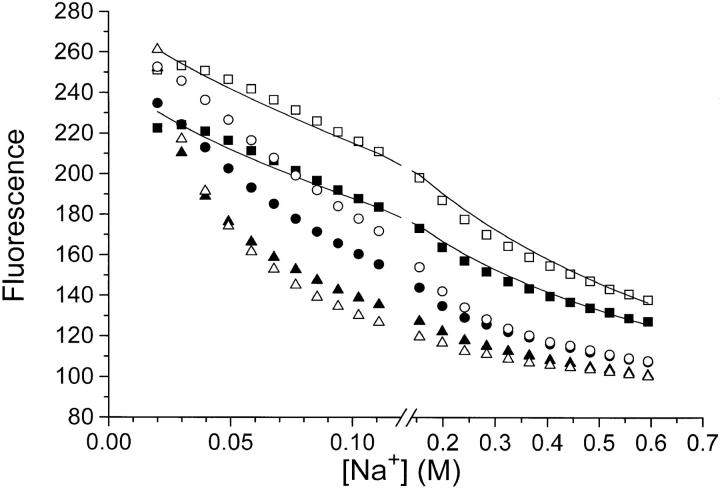
Figure 11.
Titration of eosin (▪, •, ▴) and 6-carboxyeosin (□, ○, ▵) fluorescence with NaCl (▪, □), NaNO3 (•, ○), or NaClO4 (▴, ▵). Na,K-ATPase, 0.08 mg · ml−1, in 10 mM histidine/HCl-buffer (pH = 7.0), 1 mM CDTA, 20 mM NaCl, 0.5 μM eosin, or 6-carboxyeosin was titrated with additional Na+ salts (the basal ionic strength was ≃27 mM). The fluorescence in the presence of 0.1 mM ADP (where all bound eosin or 6-carboxyeosin is displaced from the enzyme [Esmann, 1991; 1992]) is set to 100. Note that the fluorescence-data points have not been corrected for the dilution caused by the addition of Na+ salts (initial vol 2 ml, final vol 2.5 ml). The temperature was 20°C. The lines through the Cl− data are calculated (also taking dilution effects into account) using the following assumptions: K
diss = [E]·[eo]/[E·eo] and log(K
diss/ K
0) = −z
E·z
eo
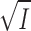 (cf. Eq. 8); [E] + [E·eo] = 0.2 μM; [eo] + [E·eo] = 0.5 μM. The fluorescence quantum yield for E·eo is about five times that of free eo and is assumed to be independent of the ionic strength, and K
0 is taken as 0.2 μM for eosin, and 0.1 μM for carboxyeosin (see Esmann and Fedosova, 1997). The curves correspond to z
E·z
eo = −1.5 (eosin) or −1.7 (carboxyeosin).
(cf. Eq. 8); [E] + [E·eo] = 0.2 μM; [eo] + [E·eo] = 0.5 μM. The fluorescence quantum yield for E·eo is about five times that of free eo and is assumed to be independent of the ionic strength, and K
0 is taken as 0.2 μM for eosin, and 0.1 μM for carboxyeosin (see Esmann and Fedosova, 1997). The curves correspond to z
E·z
eo = −1.5 (eosin) or −1.7 (carboxyeosin).