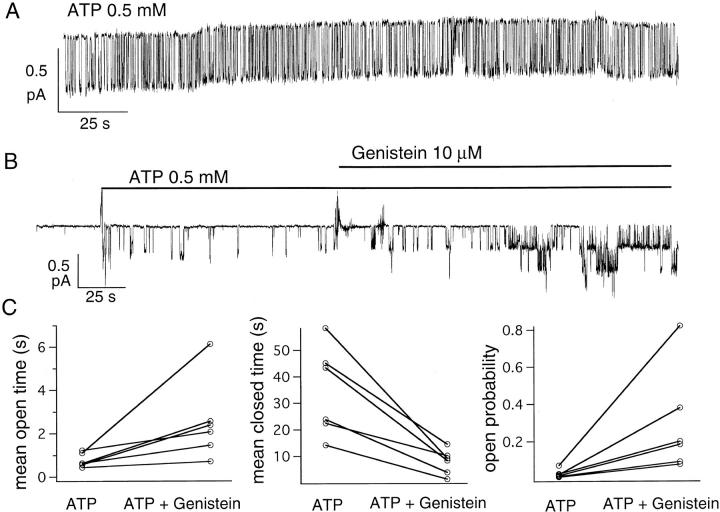
Figure 10.
The potentiation effect of genistein depends on the phosphorylation level of CFTR. (A) Typical gating behavior of a single, strongly phosphorylated, CFTR channel in an excised inside-out patch from NIH3T3 cells stimulated with forskolin plus calyculin A. (B) ATP-dependent gating of partially phosphorylated CFTR and effects of genistein on partially phosphorylated CFTR. In NIH3T3 cells, channels were activated by 50 nM forskolin to achieve partial phosphorylation. After excision, 10 μM genistein enhanced the ATP-dependent CFTR activity by approximately sevenfold. (C) Paired open times, closed times, and open probabilities from six different experiments in the presence of 0.5 mM ATP and 0.5 mM ATP plus 10 μM genistein. Although there is a large variation from experiment to experiment, a net increase in mean open time, decrease in mean closed time, and increase in P o was seen in all experiments (six of six).