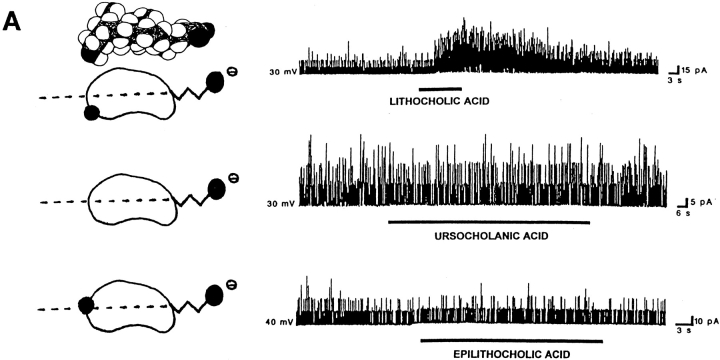
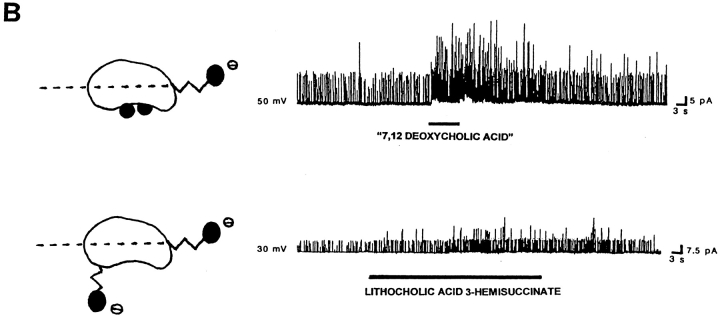
Figure 6.
The location of polar groups in the side of the steroid nucleus opposite to where the hydrophobic methyl groups are located favors the activation of BKCa channels by bile acids. For each panel, records of channel activity in inside-out patches in the absence and presence of a given bile acid analogue (100 μM) are shown on the right. A shorthand representation of the analogue applied is on the left. In the representations, the dotted line crossing these bean-shaped molecules illustrates the equatorial plane, and black circles correspond to polar groups (whether hydroxyls or carboxyls). The lateral chain and the methylenes of the hemisuccinate are depicted as zig-zag lines. For lithocholic acid, the Stuart-Briegleb space filling model also is shown at the top of the corresponding shorthand representation. (A) Lithocholic acid rapidly increases BKCa channel activity (top trace), whereas ursocholanic acid, which does not have any hydroxyl groups (middle trace), or the β-isomer of lithocholic acid (bottom trace) fail to do so. The top and middle records were obtained in the same patch, with the patch potential set to +30 mV. The bottom record was obtained with the patch potential set to +40 mV. (B) Either “7,12 deoxycholic acid” (top trace) or lithocholic acid 3-hemisuccinate (bottom trace) reversibly increase BKCa channel activity. These two compounds do not have a hydroxyl in the α configuration at C3, but still possess polar groups (whether hydroxyls or a bulky hemisuccinate) in the axial plane of the molecule, opposite to the hydrophobic side of the steroid plane (see Fig. 1 and models of these analogues on the left of each record). The patch potential was set to +50 and +30 mV.