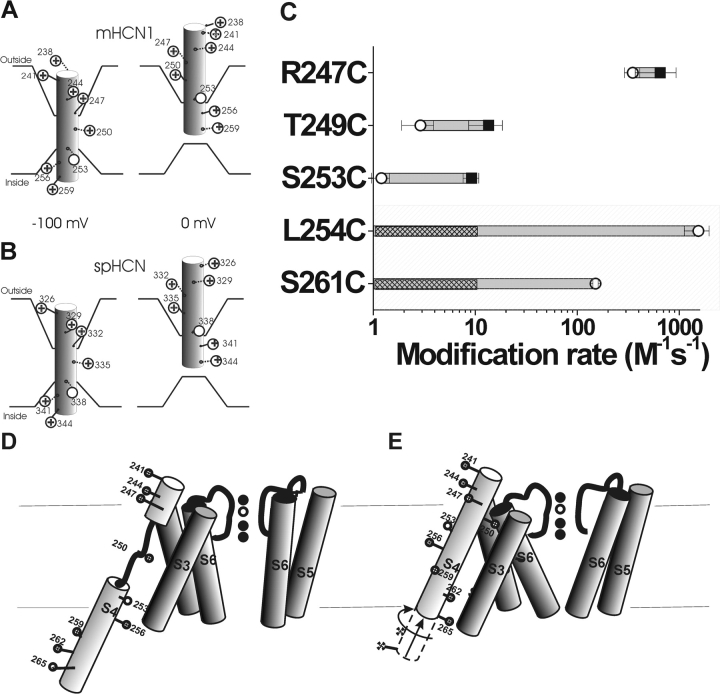
Figure 7.
A model of the voltage-dependent movements of S4 in the HCN1 channel (A) and the spHCN channel (B). (A) Based on results from this study, S4 is the voltage sensor in the HCN1 channel. HCN1 S4 is in an inward position at −100 mV, which opens the channel gate. Stepping the voltage to 0 mV results in the outward movement of S4 into the extracellular solution, causing the channel gate to close. This movement is similar to that described for the spHCN channel (Männikkö et al., 2002), seen in B. The intracellular border of S4 movement is not well-defined from our results. (C) Modification rates for cysteine mutants in the HCN1 channel in the open (○) and closed (▪) states. The shaded area represents residues tested for intracellular accessibility by excised patch recordings. The nonshaded area represents residues tested for extracellular accessibility by two-electrode voltage clamp. We did not see any modification resulting from the intracellular application of MTSEA at 0 mV; hence, we can only give an upper estimate of the modification rate for these residues. The hatch bars indicate the range of possible modification rates for these residues at 0 mV. (D and E) An alternative model for S4 movement in HCN1 channels. At hyperpolarized potentials (D), the S4 α helix unwinds external to the S253 as it moves inwards. At depolarized potentials (E), the lower portion of S4 moves outwards and S4 undergoes a conformational change into a continuous α helix.