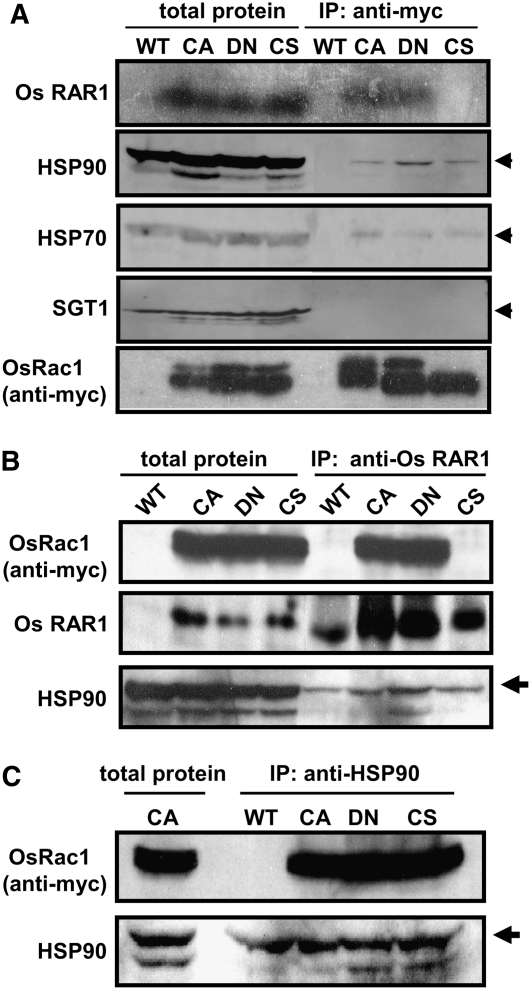
Figure 3.
Os Rac1 Forms a Complex with RAR1, HSP90, and HSP70 in Vivo.
(A) Co-IP of OsRac1 and RAR1, HSP90, and HSP70. Total protein extracts from Os Rac1 transgenic mutants were incubated with anti-myc antibody and protein A Sepharose beads. Precipitates were washed, collected by centrifugation, and separated by SDS-PAGE. Total extracted and immunoprecipitated samples from wild type cell culture were used as a control. Immunoblot analyses were performed with anti-RAR1 antibody. Os RAR1 was detected in CA and DN-OsRac1 immune complexes but not in C212S-OsRac1 (top panel). All three Os Rac1 mutants contained HSP90 (second panel). HSP70 was also detected in all three Os Rac1 mutants. Os SGT1 was not detected in Os Rac1 protein complexes (bottom panel).
(B) Immunoprecipitation with anti-RAR1 antibody and immunobloting with Os Rac1 indicates the association of RAR1 and Rac1 in complexes from CA- and DN-OsRac1 cells but not from CS-OsRac1 cells. Signals were also detected from crude extracts of transgenic but not of the nontransgenic wild-type cell culture (top panel). As a positive control, RAR1 was detected in crude extracts as well as in precipitated complexes from all cell cultures (middle panel).
(C) Confirmation of HSP90 association with Rac1 in vivo. Immunoprecipitation was performed with anti-HSP90 antibody and immunoblotted with anti-Os Rac1 (top panel) and anti-HSP90 (bottom panel) antibodies.