Abstract
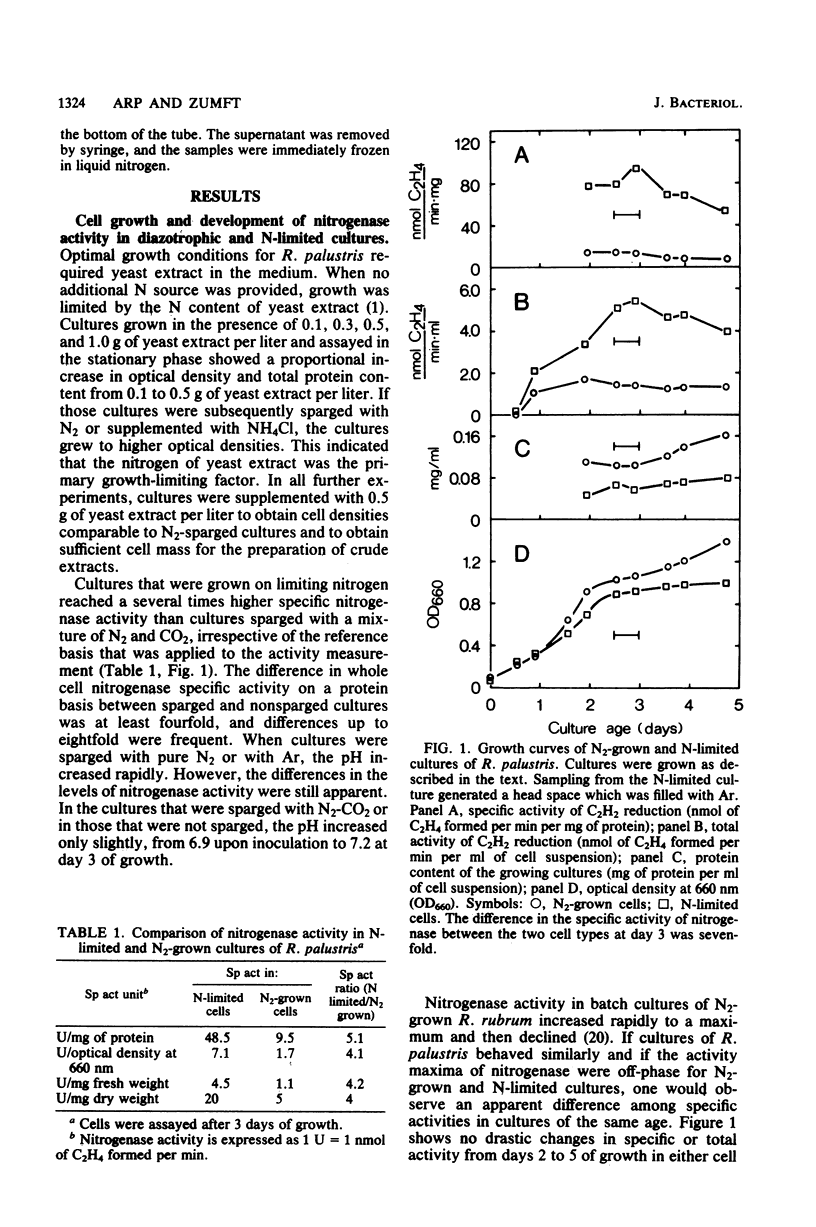
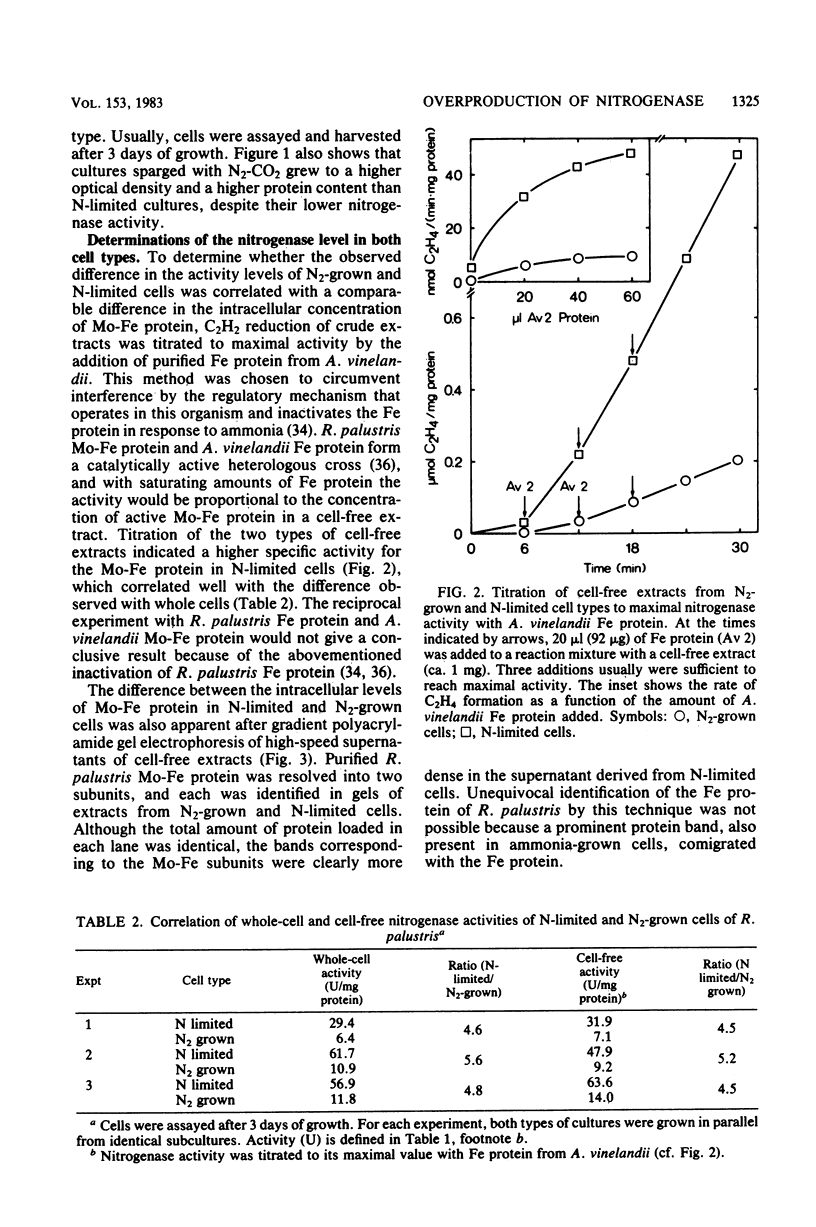
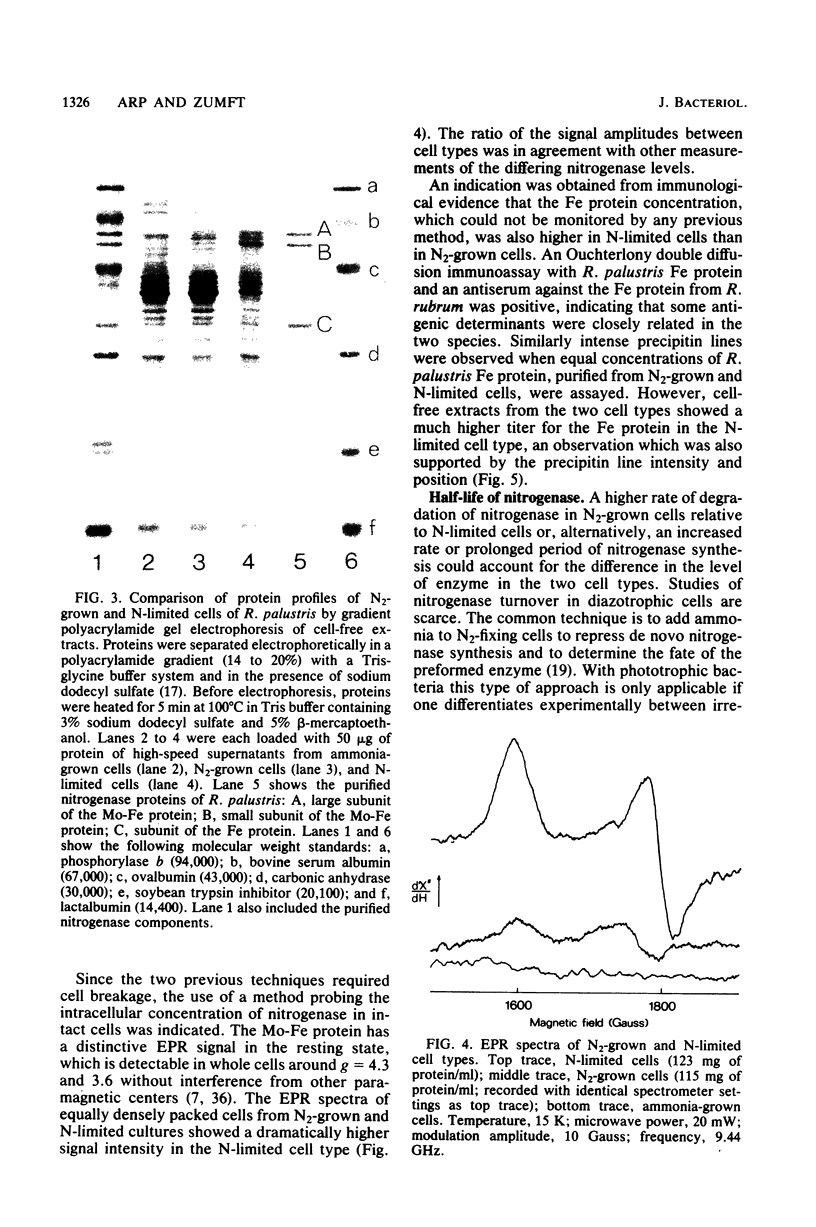
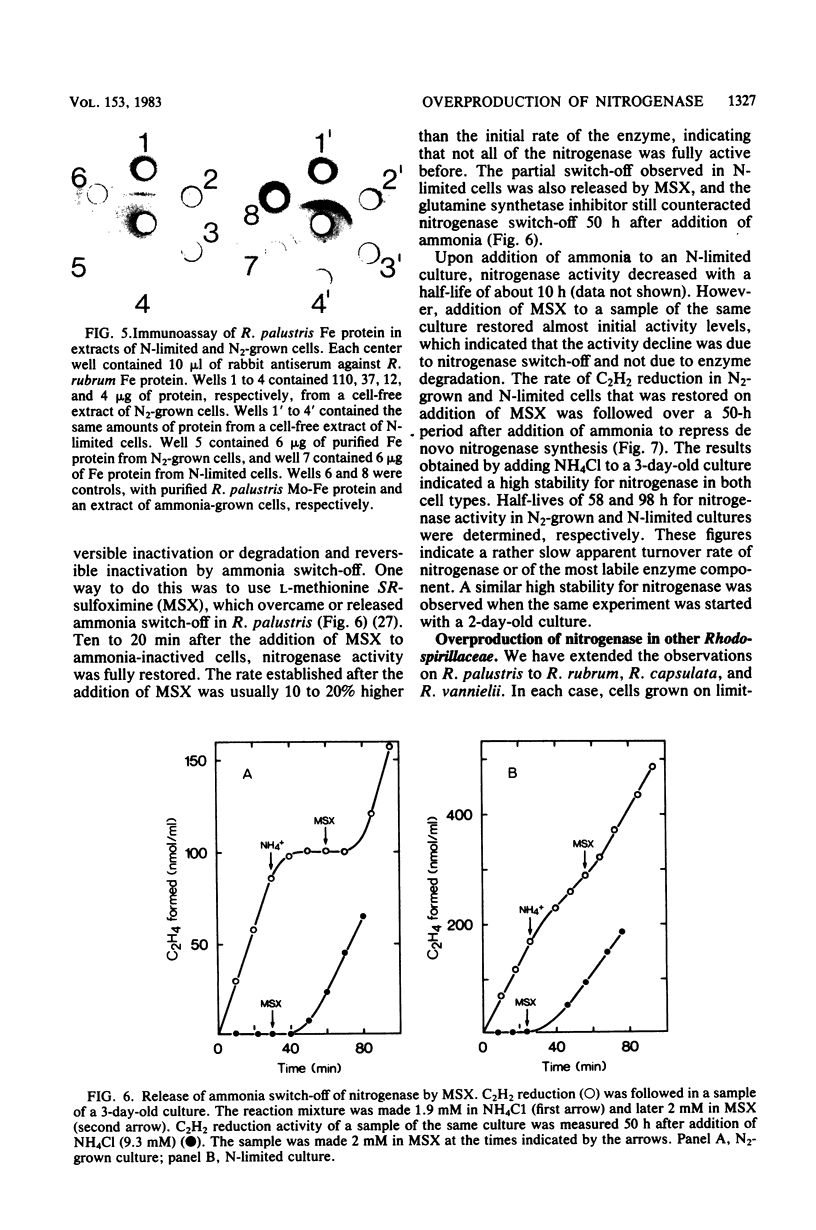
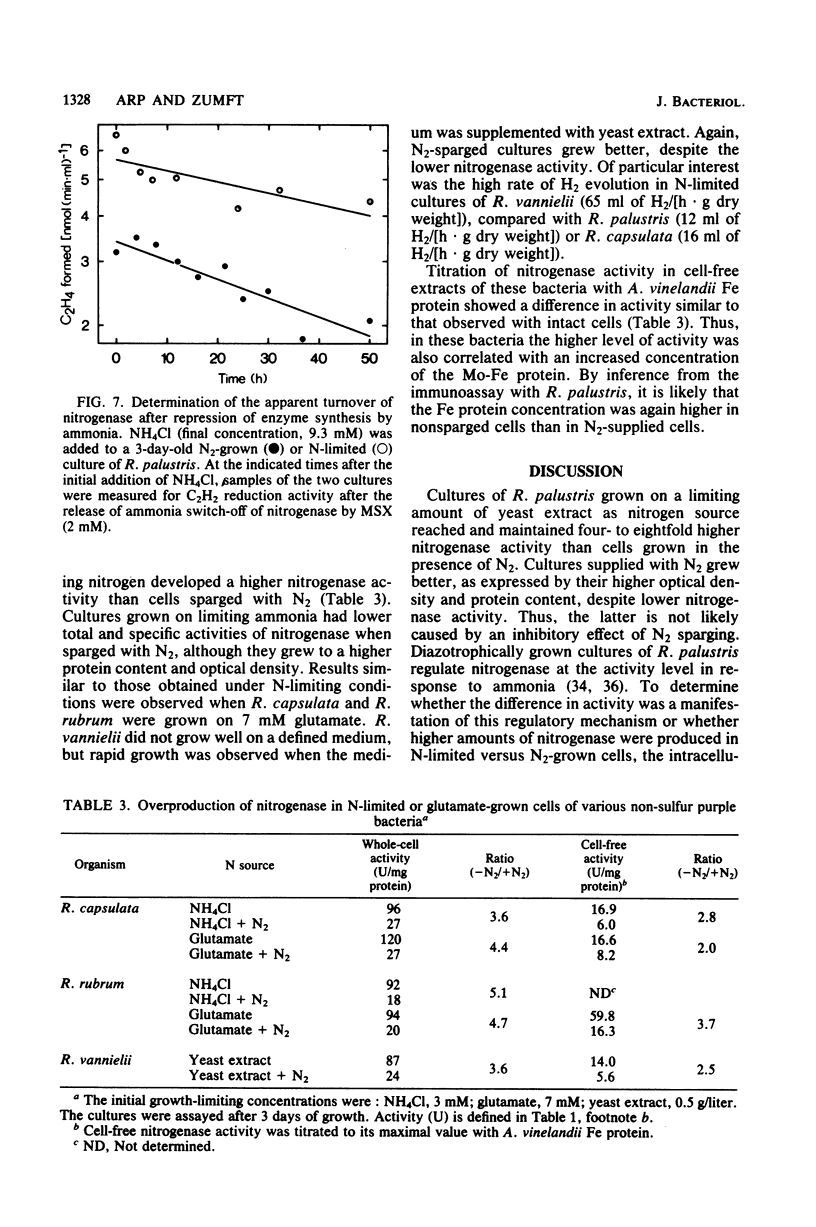
Rhodopseudomonas palustris cells grown on limiting nitrogen produced four- to eightfold higher nitrogenase specific activity relative to cells sparged with N2. The high activity of N-limited cells was the result of overproduction of the nitrogenase proteins. This was shown by four independent techniques: (i) titration of the Mo-Fe protein in cell-free extracts with Fe protein from Azotobacter vinelandii; (ii) direct detection of the subunits of Mo-Fe protein by sodium dodecyl sulfate-polyacrylamide gel electrophoresis; (iii) monitoring of the electron paramagnetic resonance spectrum of Mo-Fe protein in whole cells; and (iv) immunological assay of the Fe protein level with an antiserum against the homologous protein of Rhodospirillum rubrum. The derepressed level of nitrogenase found in N2-grown cells was not due to an increased turnover of nitrogenase. The apparent half-lives of nitrogenase in N2-grown and N-limited cells were 58 and 98 h, respectively, but were too long to account for the difference in enzyme level. Half-lives were determined by measuring nitrogenase after repression of de novo synthesis by ammonia and subsequent release of nitrogenase switch-off by methionine sulfoximine. Observations were extended to R. rubrum, Rhodopseudomonas capsulata, and Rhodomicrobium vannielii and indicated that overproduction of nitrogenase under nitrogen limitation is not an exceptional property of R. palustris, but rather a general property of phototrophic bacteria.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brenchley J. E., Baker C. A., Patil L. G. Regulation of the ammonia assimilatory enzymes in Salmonella typhimurium. J Bacteriol. 1975 Oct;124(1):182–189. doi: 10.1128/jb.124.1.182-189.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill W. J. Biochemical genetics of nitrogen fixation. Microbiol Rev. 1980 Sep;44(3):449–467. doi: 10.1128/mr.44.3.449-467.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan-Wollaston V., Cannon M. C., Beynon J. L., Cannon F. C. Role of the nifA gene product in the regulation of nif expression in Klebsiella pneumoniae. Nature. 1981 Dec 24;294(5843):776–778. doi: 10.1038/294776a0. [DOI] [PubMed] [Google Scholar]
- Daesch G., Mortenson L. E. Effect of ammonia on the synthesis and function of the N 2 -fixing enzyme system in Clostridium pasteurianum. J Bacteriol. 1972 Apr;110(1):103–109. doi: 10.1128/jb.110.1.103-109.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L. C., Shah V. K., Brill W. J., Orme-Johnson W. H. Nitrogenase. II. Changes in the EPR signal of component I (iron-molybdenum protein) of Azotobacter vinelandii nitrogenase during repression and derepression. Biochim Biophys Acta. 1972 Feb 28;256(2):512–523. doi: 10.1016/0005-2728(72)90079-5. [DOI] [PubMed] [Google Scholar]
- Hillmer P., Gest H. H2 metabolism in the photosynthetic bacterium Rhodopseudomonas capsulata: H2 production by growing cultures. J Bacteriol. 1977 Feb;129(2):724–731. doi: 10.1128/jb.129.2.724-731.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillmer P., Gest H. H2 metabolism in the photosynthetic bacterium Rhodopseudomonas capsulata: production and utilization of H2 by resting cells. J Bacteriol. 1977 Feb;129(2):732–739. doi: 10.1128/jb.129.2.732-739.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kustu S. G., McFarland N. C., Hui S. P., Esmon B., Ames G. F. Nitrogen control of Salmonella typhimurium: co-regulation of synthesis of glutamine synthetase and amino acid transport systems. J Bacteriol. 1979 Apr;138(1):218–234. doi: 10.1128/jb.138.1.218-234.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MacNeil D., Brill W. J. Mutations in nif genes that cause Klebsiella pneumoniae to be derepressed for nitrogenase synthesis in the presence of ammonium. J Bacteriol. 1980 Nov;144(2):744–751. doi: 10.1128/jb.144.2.744-751.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortenson L. E. Regulation of nitrogen fixation. Curr Top Cell Regul. 1978;13:179–232. doi: 10.1016/b978-0-12-152813-3.50010-0. [DOI] [PubMed] [Google Scholar]
- Neilson A. H., Nordlund S. Regulation of nitrogenase synthesis in intact cells of Rhodospirillum rubrum: inactivation of nitrogen fixation by ammonia, L-glutamine and L-asparagine. J Gen Microbiol. 1975 Nov;91(1):53–62. doi: 10.1099/00221287-91-1-53. [DOI] [PubMed] [Google Scholar]
- Postgate J. R., Krishnapillai V. Expression of Klebsiella nif and his genes in Salmonella typhimurium. J Gen Microbiol. 1977 Feb;98(2):379–385. doi: 10.1099/00221287-98-2-379. [DOI] [PubMed] [Google Scholar]
- Ruvkun G. B., Ausubel F. M. Interspecies homology of nitrogenase genes. Proc Natl Acad Sci U S A. 1980 Jan;77(1):191–195. doi: 10.1073/pnas.77.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer S., Kerfin W., Böger P. Increase of nitrogenase activity in the blue-green alga Nostoc muscorum (Cyanobacterium). J Bacteriol. 1980 Dec;144(3):1017–1023. doi: 10.1128/jb.144.3.1017-1023.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah V. K., Brill W. J. Nitrogenase. IV. Simple method of purification to homogeneity of nitrogenase components from Azotobacter vinelandii. Biochim Biophys Acta. 1973 May 30;305(2):445–454. doi: 10.1016/0005-2728(73)90190-4. [DOI] [PubMed] [Google Scholar]
- Sweet W. J., Burris R. H. Inhibition of nitrogenase activity by NH+4 in Rhodospirillum rubrum. J Bacteriol. 1981 Feb;145(2):824–831. doi: 10.1128/jb.145.2.824-831.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upchurch R. G., Mortenson L. E. In vivo energetics and control of nitrogen fixation: changes in the adenylate energy charge and adenosine 5'-diphosphate/adenosine 5'-triphosphate ratio of cells during growth on dinitrogen versus growth on ammonia. J Bacteriol. 1980 Jul;143(1):274–284. doi: 10.1128/jb.143.1.274-284.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall J. D., Gest H. Derepression of nitrogenase activity in glutamine auxotrophs of Rhodopseudomonas capsulata. J Bacteriol. 1979 Mar;137(3):1459–1463. doi: 10.1128/jb.137.3.1459-1463.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weare N. M., Shanmugam K. T. Photoproduction of ammonium ion from N2 in Rhodospirillum rubrum. Arch Microbiol. 1976 Nov 2;110(23):207–213. doi: 10.1007/BF00690229. [DOI] [PubMed] [Google Scholar]
- Wei G. R., Kustu S. Glutamine auxotrophs with mutations in a nitrogen regulatory gene, ntrC, that is near glnA. Mol Gen Genet. 1981;183(2):392–399. doi: 10.1007/BF00270646. [DOI] [PubMed] [Google Scholar]
- Zumft W. G., Castillo F. Regulatory properties of the nitrogenase from Rhodopseudomonas palustris. Arch Microbiol. 1978 Apr 27;117(1):53–60. doi: 10.1007/BF00689351. [DOI] [PubMed] [Google Scholar]
- de Bruijn F. J., Ausubel F. M. The cloning and transposon Tn5 mutagenesis of the glnA region of Klebsiella pneumoniae: identification of glnR, a gene involved in the regulation of the nif and hut operons. Mol Gen Genet. 1981;183(2):289–297. doi: 10.1007/BF00270631. [DOI] [PubMed] [Google Scholar]
- de Vasconcelos L., Fay P. Nitrogen metabolism and ultrastructure in Anabaena cylindrica. I. The effect of nitrogen starvation. Arch Mikrobiol. 1974 Mar 28;96(4):271–279. [PubMed] [Google Scholar]