Abstract
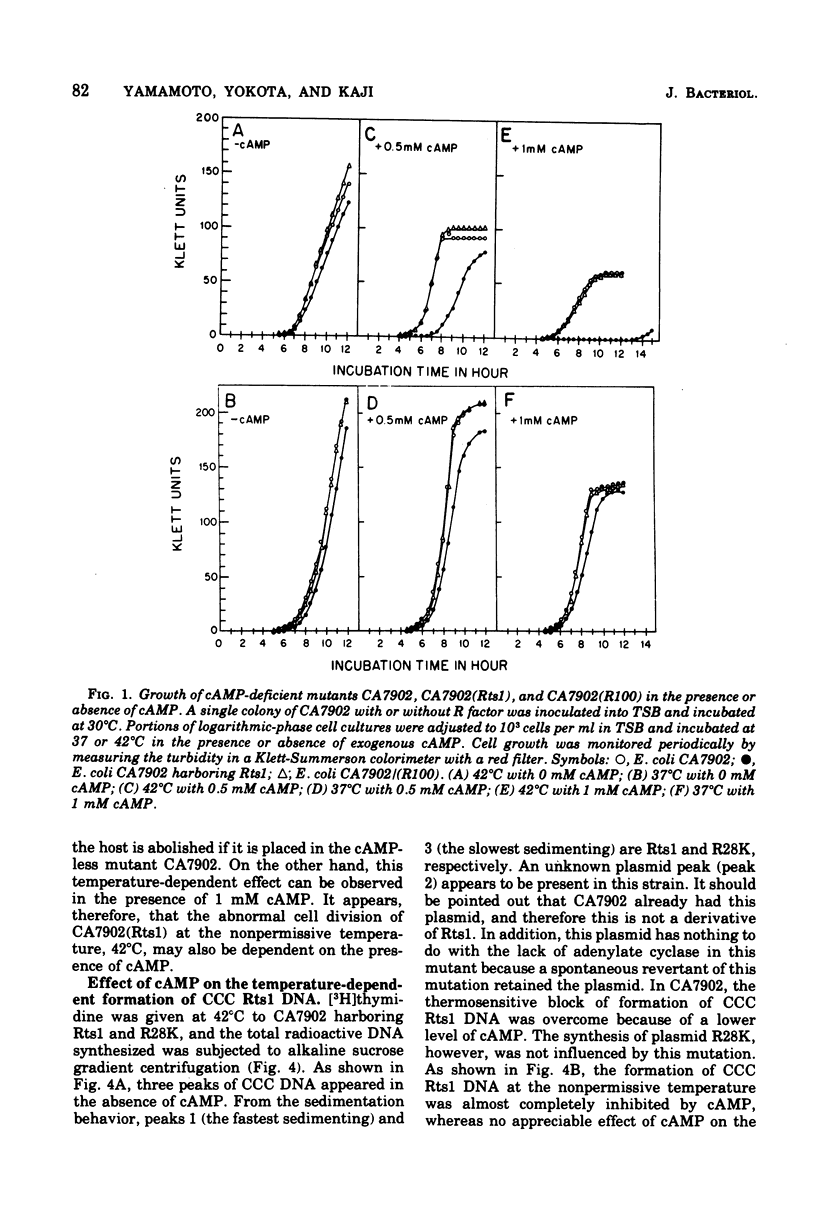
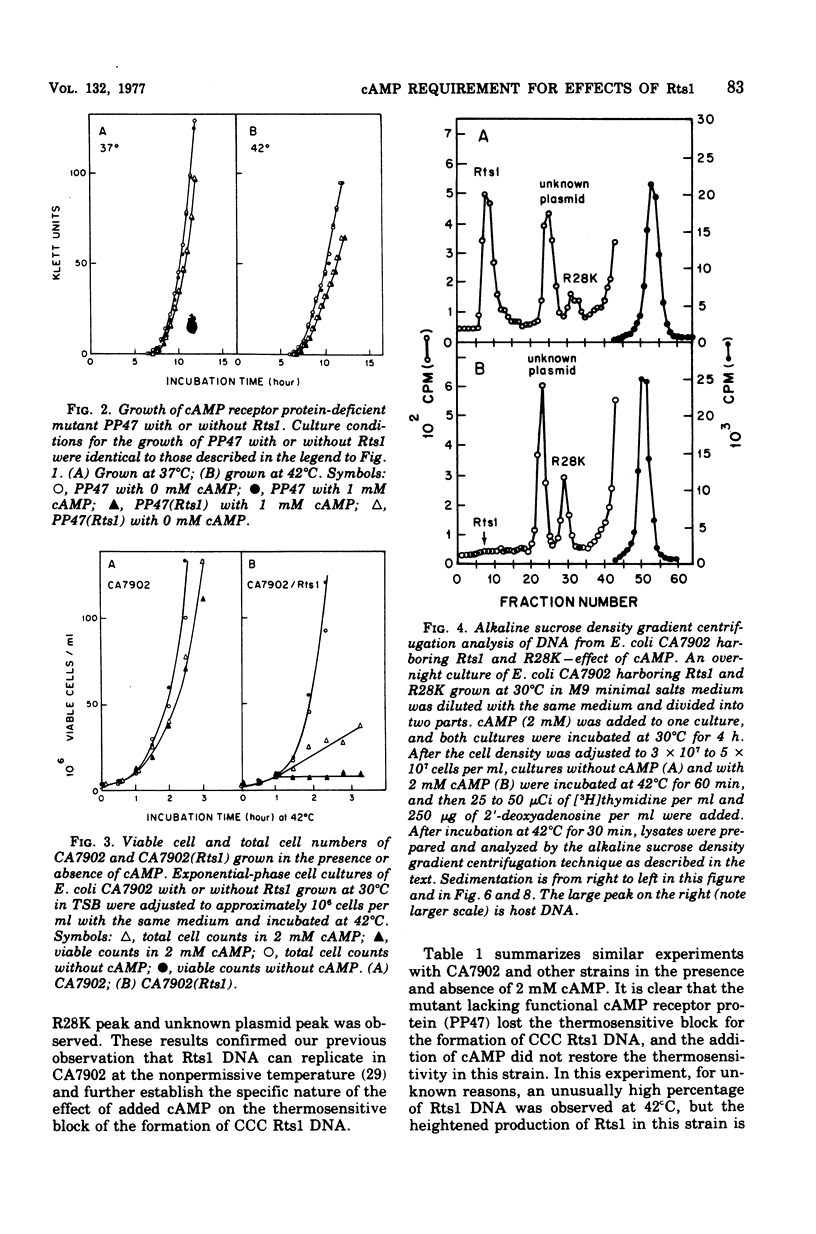
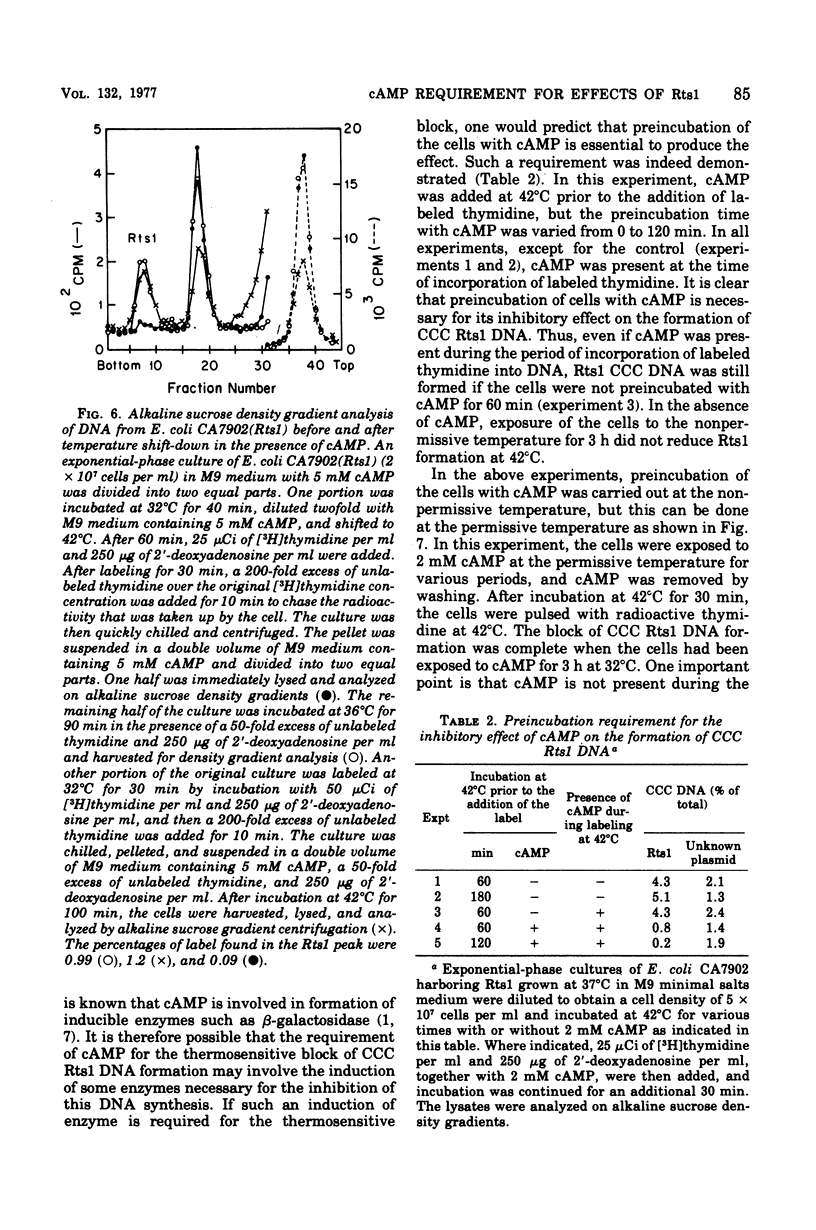
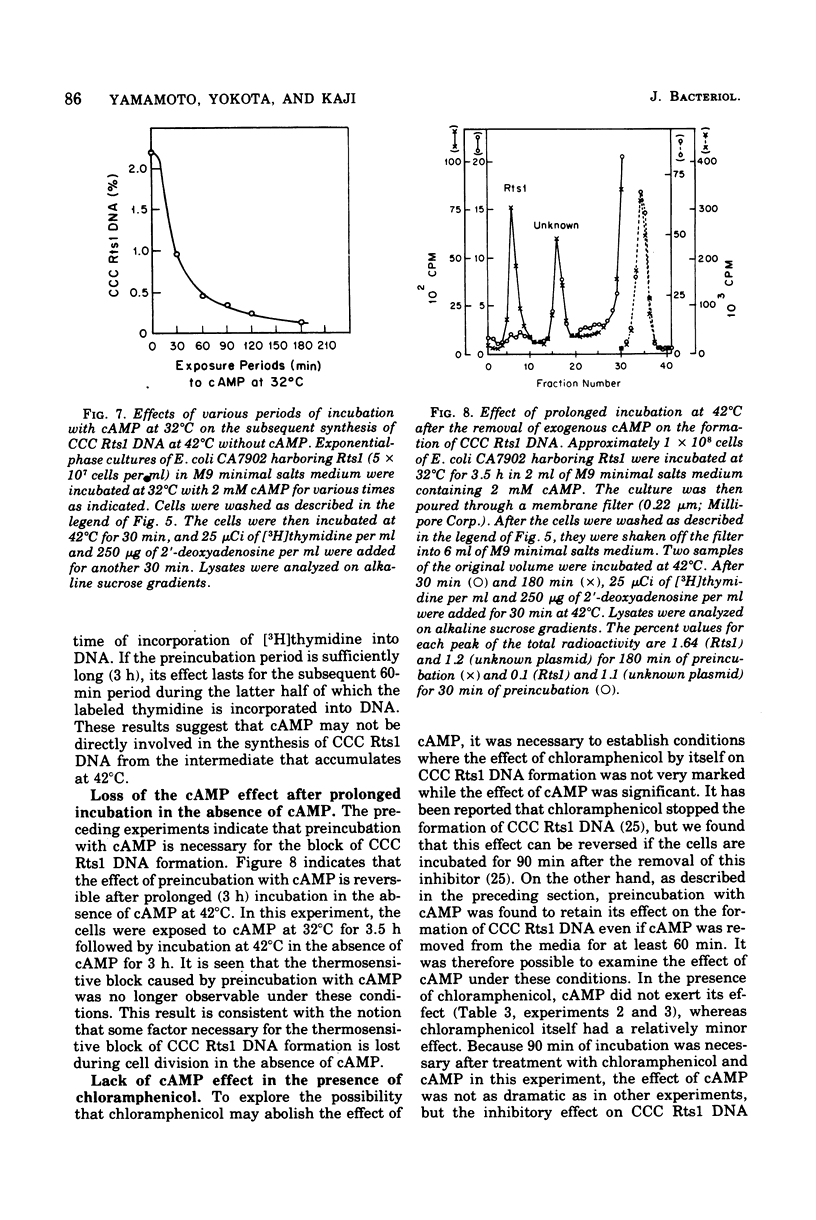
Previous publications showed that a covalently closed circular (CCC) Rts1 plasmid deoxyribonucleic acid (DNA) that confers kanamycin resistance upon the host bacteria inhibits host growth at 42°C but not at 32°C. At 42°C, the CCC Rts1 DNA is not formed, and cells without plasmids emerge. To investigate the possible role of cyclic adenosine 3′,5′-monophosphate (cAMP) in the action of Rts1 on host bacteria, Rts1 was placed in an Escherichia coli mutant (CA7902) that lacks adenylate cyclase or in E. coli PP47 (a mutant lacking cAMP receptor protein). Rts1 did not exert the thermosensitive effect on these cells, and CCC Rts1 DNA was formed even at 42°C. Upon addition of cAMP to E. coli CA7902(Rts1), cell growth and formation of CCC Rts1 DNA were inhibited at 42°C. The addition of cAMP to E. coli PP47(Rts1) did not cause inhibitory effects on either cell growth or CCC Rts1 DNA formation at 42°C. The inhibitory effect of cAMP on E. coli CA7902(Rts1) is specific to this cyclic nucleotide, and other cyclic nucleotides such as cyclic guanosine 3′,5′-monophosphate did not have the effect. For this inhibitory effect, cells have to be preincubated with cAMP; the presence of cAMP at the time of CCC Rts1 DNA formation is not enough for the inhibitory effect. If the cells are preincubated with cAMP, one can remove cAMP during the [3H]thymidine pulse and still observe its inhibitory effect on the formation of CCC Rts1 DNA. The presence of chloramphenicol during this preincubation period abolished the inhibitory effect of cAMP. These observations suggest that cAMP is necessary to induce synthesis of a protein that inhibits CCC Rts1 DNA formation and cell growth at 42°C.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- De Crombrugghe B., Chen B., Anderson W., Nissley P., Gottesman M., Pastan I., Perlman R. Lac DNA, RNA polymerase and cyclic AMP receptor protein, cyclic AMP, lac repressor and inducer are the essential elements for controlled lac transcription. Nat New Biol. 1971 Jun 2;231(22):139–142. doi: 10.1038/newbio231139a0. [DOI] [PubMed] [Google Scholar]
- De Crombrugghe B., Pastan I., Shaw W. V., Rosner J. L. Stimulation by cyclic AMP and ppGpp of chloramphenicol acetyl transferase synthesis. Nat New Biol. 1973 Feb 21;241(112):237–239. doi: 10.1038/newbio241237a0. [DOI] [PubMed] [Google Scholar]
- DiJoseph C. G., Bayer M. E., Kaji A. Host cell growth in the presence of the thermosensitive drug resistance factor, Rts1. J Bacteriol. 1973 Jul;115(1):399–410. doi: 10.1128/jb.115.1.399-410.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiJoseph C. G., Kaji A. Molecular nature of the deoxyribonucleic acid of a thermosensitive R factor, Rts1. J Bacteriol. 1974 Dec;120(3):1364–1369. doi: 10.1128/jb.120.3.1364-1369.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiJoseph C. G. The thermosensitive lesion in the replication of the drug resistance factor, Rts1. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2515–2519. doi: 10.1073/pnas.71.6.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dottin R. P., Shiner L. S., Hoar D. I. Adenosine 3',5'-cyclic monophosphate regulation of chloramphenicol acetyltransferase synthesis in vitro from P1CM DNA. Virology. 1973 Feb;51(2):509–511. doi: 10.1016/0042-6822(73)90453-4. [DOI] [PubMed] [Google Scholar]
- Emmer M., deCrombrugghe B., Pastan I., Perlman R. Cyclic AMP receptor protein of E. coli: its role in the synthesis of inducible enzymes. Proc Natl Acad Sci U S A. 1970 Jun;66(2):480–487. doi: 10.1073/pnas.66.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eron L., Arditti R., Zubay G., Connaway S., Beckwith J. R. An adenosine 3':5'-cyclic monophosphate-binding protein that acts on the transcription process. Proc Natl Acad Sci U S A. 1971 Jan;68(1):215–218. doi: 10.1073/pnas.68.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood C. R., Meynell E. Cyclic AMP and the production of sex pili by E. coli K-12 carrying derepressed sex factors. Nature. 1975 Apr 17;254(5501):628–660. doi: 10.1038/254628a0. [DOI] [PubMed] [Google Scholar]
- Harwood J., Smith D. H. Catabolite repression of chloramphenicol acetyl transferase synthesis in E. coli K12. Biochem Biophys Res Commun. 1971 Jan 8;42(1):57–62. doi: 10.1016/0006-291x(71)90361-5. [DOI] [PubMed] [Google Scholar]
- Katz L., Helinski D. R. Effect of inhibitors of ribonucleic acid and protein synthesis on the cyclic adenosine monophosphate stimulation of plasmid ColE1 replication. J Bacteriol. 1974 Aug;119(2):450–460. doi: 10.1128/jb.119.2.450-460.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L., Kingsbury D. T., Helinski D. R. Stimulation by cyclic adenosine monophosphate of plasmid deoxyribonucleic acid replication and catabolite repression of the plasmid deoxyribonucleic acid-protein relaxation complex. J Bacteriol. 1973 May;114(2):577–591. doi: 10.1128/jb.114.2.577-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontomichalou P., Mitani M., Clowes R. C. Circular R-factor molecules controlling penicillinase synthesis, replicating in Escherichia coli under either relaxed or stringent control. J Bacteriol. 1970 Oct;104(1):34–44. doi: 10.1128/jb.104.1.34-44.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S. Properties of adenyl cyclase and cyclic adenosine 3',5'-monophosphate receptor protein-deficient mutants of Escherichia coli. J Bacteriol. 1976 Feb;125(2):545–555. doi: 10.1128/jb.125.2.545-555.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupersztoch-Portnoy Y. M., Miklos G. L., Helinski D. R. Properties of the relaxation complexes of supercoiled deoxyribonucleic acid and protein of the R plasmids R64, R28K, and R6K. J Bacteriol. 1974 Oct;120(1):545–548. doi: 10.1128/jb.120.1.545-548.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller Z., Varmus H. E., Parks J. S., Perlman R. L., Pastan I. Regulation of gal messenger ribonucleic acid synthesis in Escherichia coli by 3',5'-cyclic adenosine monophosphate. J Biol Chem. 1971 May 10;246(9):2898–2903. [PubMed] [Google Scholar]
- Mizuno T., Yamada H., Yamagata H., Mizushima S. Coordinated alterations in ribosomes and cytoplasmic membrane in sucrose-dependent, spectinomycin-resistant mutants of Escherichia coli. J Bacteriol. 1976 Feb;125(2):524–530. doi: 10.1128/jb.125.2.524-530.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa A., Tamada T. Involvement of cyclic 3',5'-adenosine monophosphate in replication of colicinogenic factor E 1 DNA. Biochem Biophys Res Commun. 1972 Nov 15;49(4):977–983. doi: 10.1016/0006-291x(72)90308-7. [DOI] [PubMed] [Google Scholar]
- Nakazawa A., Tamada T. Stimulation of colicin E 1 synthesis by cyclic 3', 5'-adenosine monophosphate in mitomycin C-induced Escherichia coli. Biochem Biophys Res Commun. 1972 Jan 31;46(2):1004–1010. doi: 10.1016/s0006-291x(72)80241-9. [DOI] [PubMed] [Google Scholar]
- Nakazawa T., Yokota T. Requirement of adenosine-3',5'-cyclic monophosphate for L-arabinose isomerase synthesis in Escherichia coli. J Bacteriol. 1973 Mar;113(3):1412–1418. doi: 10.1128/jb.113.3.1412-1418.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler J., Adelberg E. A. Temperature dependence of sex-factor maintenance in Escherichia coli K-12. J Bacteriol. 1972 Jan;109(1):447–449. doi: 10.1128/jb.109.1.447-449.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terawaki Y., Kakizawa Y., Takayasu H., Yoshikawa M. Temperature sensitivity of cell growth in Escherichia coli associated with the temperature sensitive R(KM) factor. Nature. 1968 Jul 20;219(5151):284–285. doi: 10.1038/219284a0. [DOI] [PubMed] [Google Scholar]
- Terawaki Y., Rownd R., Nakaya R. Effects of inhibition and restoration of protein synthesis on the replication of the R factor Rts1 in Proteus mirabilis. J Bacteriol. 1974 Feb;117(2):687–695. doi: 10.1128/jb.117.2.687-695.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terawaki Y., Rownd R. Replication of the R factor Rts1 in Proteus mirabilis. J Bacteriol. 1972 Feb;109(2):492–498. doi: 10.1128/jb.109.2.492-498.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terawaki Y., Takayasu H., Akiba T. Thermosensitive replication of a kanamycin resistance factor. J Bacteriol. 1967 Sep;94(3):687–690. doi: 10.1128/jb.94.3.687-690.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata H., Uchida H. Chromosomal mutations affecting the stability of sex-factors in Escherichia coli. J Mol Biol. 1972 Jan 28;63(2):281–294. doi: 10.1016/0022-2836(72)90375-0. [DOI] [PubMed] [Google Scholar]
- Yamagata H., Uchida H. Spectinomycin resistance mutations affecting the stability of sex-factors in Escherichia coli. J Mol Biol. 1972 Jun 28;67(3):533–535. doi: 10.1016/0022-2836(72)90472-x. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Yokota T., Kaji A. The role of cyclic AMP in the thermosensitive lesion of the formation of closed covalent circular Rts 1 DNA. Biochem Biophys Res Commun. 1975 Feb 3;62(3):546–552. doi: 10.1016/0006-291x(75)90433-7. [DOI] [PubMed] [Google Scholar]
- Yokota T., Gots J. S. Requirement of adenosine 3', 5'-cyclic phosphate for flagella formation in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1970 Aug;103(2):513–516. doi: 10.1128/jb.103.2.513-516.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T., Kasuga T., Kaneko M., Kuwahara S. Genetic behavior of R factors in Vibrio cholerae. J Bacteriol. 1972 Jan;109(1):440–442. doi: 10.1128/jb.109.1.440-442.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T., Kasuga T. Requirement of adenosine 3',5'-cyclic phosphate for formation of the phage lambda receptor in Escherichia coli. J Bacteriol. 1972 Mar;109(3):1304–1306. doi: 10.1128/jb.109.3.1304-1306.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


