Abstract
Neutrophils express only two intermediate filament proteins, vimentin and, to a lesser extent, lamin B. Lamin B mutant mice die shortly after birth; however, mice lacking vimentin (vim−/−) develop and reproduce normally. Herein, we investigate for the first time the role of vimentin in general inflammation in vivo and in neutrophil functions ex vivo. Using the murine air pouch model, we show that the inflammatory response induced by lipopolysaccharide, interleukin-21 or carageenan is, intriguingly, uncompromised in vim−/− mice and that neutrophil functions are not altered ex vivo. Our results suggest that vimentin is dispensable for the establishment of an acute inflammatory response in vivo. In addition, based on several criteria presented in this study, one has to accept the existence of a very complex compensatory mechanism to explain the intriguing normal inflammatory response in absence of vimentin.
Keywords: air pouch, inflammation, knockout mice, neutrophils, vimentin
Introduction
Only two intermediate filament (IF) proteins are known to be expressed in neutrophils: vimentin and, to a lesser extent, lamin B (B1, primarily, and B2) [1] in contrast to lamin B1 mutant mice, which die at birth due to defect lungs and bone defects [2]. Collucci-Guyon and collaborators demonstrated that vimentin-null mice (vim−/−) develop and reproduce without any apparent defects [3]. This fascinating paradigm suggests that vimentin is not required for survival and may not be of significance under normal physiological conditions. This is difficult to imagine, given the roles of vimentin in general biology. However, this does not exclude the possibility for a particular role of vimentin under certain conditions. In this regard, since the first study published by Collucci-Guyon and collaborators [3], a few studies conducted with these mice or using different cell culture models derived from vim−/− mice have indicated that vimentin can play an important role. For example, reduction of renal mass was found to be lethal in vim−/− mice, whereas no lethality was observed in littermate controls [4]; cerebellar defects and impaired motor coordination were noted in vim−/− mice; vimentin was implicated in diameter and wall mass changes during flow-induced arterial remodelling [5], and impaired wound healing in both embryonic and adult mice [6] was established using these mice. Curiously, vimentin was not essential for efficient tumour growth and differentiation in vivo [7]. The mechanical stability, migration and contractile capacity of fibroblasts derived from vim−/− mice were found to be impaired [8]. More recently, absence of vimentin was correlated with aberrant expression and distribution of intercellular cell adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule (VCAM) on endothelial cells and integrin β1 on peripheral blood mononuclear cells [9]. The results of this latter study showed that vimentin is involved in lymphocyte adhesion and transmigration. Thus, although vim−/− mice have a normal phenotype, it would appear that vimentin has specialized roles contributing to specific cellular functions which may be counteracted in vivo.
We have documented recently that several cytoskeletal proteins, including the two IF proteins vimentin and lamin B1, are cleaved via caspase activation in human apoptotic neutrophils [10–14], as well as in promyelocytes and eosinophils [15,16]. More recently, we demonstrated that among the different cytoskeletal proteins we have tested, paxillin, gelsolin, vinculin, α- and β-tubulin, only the IF protein vimentin and lamin B1 were expressed on the cell surface of apoptotic neutrophils, suggesting that neutrophils may be a source of autoantigens for the development of autoantibodies directed against vimentin and lamin B1 associated with various rheumatic diseases [17]. Furthermore, the presence of vimentin on the cell surface of apoptotic neutrophils may be involved in the recognition process of apoptotic neutrophils by phagocytes for the resolution of inflammation, although this remains to be demonstrated.
Using the murine air pouch model, we have also demonstrated that vimentin was cleaved when neutrophils harvested from lipopolysaccharide (LPS)-induced air pouches were incubated in vitro with the potent pro-apoptotic plant lectin Viscum album agglutinin-I [18]. Given the importance of vimentin in general immunology [9,19–23], inflammation and biology of neutrophils [10–14,17], and the fact that a generation of mice totally devoid of vimentin could develop and reproduce normally [3], we decided to investigate the role of vimentin during an inflammatory response in vivo and on neutrophil cell functions in vitro, two situations which have been curiously not or poorly investigated.
Methods
Chemicals, agonists and antibodies
RPMI-1640, HEPES, penicillin/streptomycin (P/S), bovine serum albumin (BSA), V. album agglutinin-1 (VAA-I), arsenic trioxide (As2O3) LPS, carrageenan, mouse monoclonal anti-vinculin antibody (clone hVIN-1), the anti-mouse vimentin antibody (clone VIM-13·2, ascites fluid), phorbol 12-myristate 13-acetate (PMA) and the calcium ionophore A23187 were purchased from Sigma-Aldrich Chemical Company (St Louis, MO, USA). The anti-lamin B1 (C-20) antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Horseradish peroxidase-conjugated antibodies were purchased from Jackson Immunoresearch (West Grove, PA, USA). Recombinant murine granulocyte–macrophage colony-stimulating factor (rmGM-CSF) and recombinant murine interleukin-21 (rmIL-21) were purchased from PeproTech Inc. (Rocky Hill, NJ, USA) and R&D Systems (Hornby, Ontario, Canada), respectively. Annexin-V-fluorescein isothiocyanate (FITC) was purchased from Biosource (Montreal, Quebec, Canada). The FITC-rat anti-mouse F4/80 antigen (rat IgG2b), the FITC-rat anti-mouse neutrophils clone 7/4 (rat IgG2a), the FITC-rat IgG2a and FITC-rat IgG2b negative controls were from Serotec Inc. (Raleigh, NC, USA). Dihydrorhodamine 123 (DHR123) was purchased from Invitrogen/Molecular Probes (Burlington, Ontario, Canada).
Mice
Vimentin-null mutant mice (Vim1 mutation) [3] and corresponding control animals were a generous gift of Alain Privat (INSERM U, Université Montpellier II, Montpellier, France). These animals were crossed with C57BL/6 in order to establish our own colony of mice. To identify wild-type mice (vim+/+), heterozygous (vim+/−) and homozygous vimentin-deficient mice (vim−/−), DNA was extracted from the tails of mice and the presence of targeted alleles was monitored by polymerase chain reaction (PCR) using the following primers: primer 1 (5′-TGTCCTCGTCCTCCTACCGC-3′); primer 2 (5′-AGCTGCTCGAGCTCAGCCAGC-3′); and primer 3 (5′-CTGTTCGCCAGGCTCAAGGC- 3′). PCR amplification was performed using Taq platinum polymerase (Invitrogen) under the conditions recommended by the supplier on a T personal model PCR machine (Biometra) using 30 cycles of denaturation at 94°C for 30 s, annealing at 66°C for 30 s and extension at 72°C for 1 min. The 1–2 primer pair allows amplification of a 398-base pairs (bp) fragment when the wild-type allele is present and the 2–3 primer pair allows amplification of a 530-bp fragment when the disrupted allele is present, as described previously [8]. Lack of vimentin was confirmed at the protein level by immunoblotting using the anti-vimentin antibody (see below for details of the immunoblotting procedures). Experiments were performed under protocols approved by Animal Use and Care Committees at INRS-Insitut Armand-Frappier.
Blood leucocyte counts
Blood collection was performed by cardiac puncture on mice under anaesthesia with isoflurane as described previously [24]. Total leucocyte counts were performed by cytology using a haemacytometer. Differential cell count was performed with an aliquot of 0·5 × 106 white blood cells cytocentrifuged onto a microscope slide and coloured with the Hema-3 stain set (Biochemical Sciences Inc., Swedesboro, NJ, USA). At least 300 leucocytes per slide were counted under light microscope. Cell viability was always ≥ 98%.
Air pouch experiments
Dorsal air pouches were raised in vim+/+, vim+/− and vim−/− mice, as described previously [25]. LPS (positive control for neutrophil attraction), IL-21 (positive control for neutrophil and mononuclear cell attraction) [25] or the diluent [phosphate-buffered saline (PBS)] was injected into the air pouches of mice 6 or 24 h before the mice were killed by CO2 asphyxiation. In some experiments, carrageenan (CRGN) was used in order to prolong leucocyte influx in vivo for a 24-h period. Supernatants were collected and stored at −20°C for further analysis. The cells were suspended in 1 ml of Hank's balanced salt solution–ethylenediamine tetraacetic acid (HBSS–EDTA) and counted. Cells (3 × 105) were centrifuged, spread onto microscope slides and stained with Hema-stain to allow quantification of granulocytic and mononuclear populations. To characterize further the leucocyte subpopulations, the cells were suspended in PBS containing 5 µg/ml human IgG for 30 min at 4°C to block Fc receptors and then stained for 30 min at 4°C with purified rat anti-mouse 7/4 monoclonal antibody (MoAb) directed against murine neutrophils or rat anti-mouse F4/80 antigen antibody directed against murine monocytes/macrophages, as published previously [25]. Analyses were performed with a fluorescence activated cell sorter (FACScan) (Becton Dickinson, San Jose, CA, USA).
Protein expression
Murine neutrophils, as well as the exudates harvested from LPS-induced air pouches, were collected from vim−/− and vim+/+ animals, and the expression of intracellular proteins was compared after running sodium dodecyl sulphate (SDS)-electrophoresis. In addition, we performed an antibody array assay (ChemiArray™ mouse inflammation antibody array I Map; Chemicon International, Temecula, CA, USA) to detect several inflammatory cytokines/chemokines, including B-lymphocyte chemoattractant (BLC), eotaxin, eotaxin-2, FasL, fractalkine, granulocyte–colony-stimulating factor (G-CSF), GM-CSF, interferon (IFN)-γ, IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-6, IL-9, IL-10, IL-12p40/p70, IL-12p70, IL-13, IL-17, IFN-inducible T cell alpha chemoattractant (I-TAC), murine GRO-alpha, leptin, lymphotactin, monocyte chemotactic protein (MCP)-1, M-CSF, monokine induced by gamma interferon (MIG), macrophage inflammatory protein (MIP)-1α, MIP-1γ, regulated on activation, normal T cell expressed and secreted (RANTES), stromal cell-derived factor (SDF)-1, T cell activation (TCA)-3, thymus-expressed chemokine (TECK), tissue inhibitor of metalloproteinase-1 (TIMP-1), TIMP-2, tumour necrosis factor (TNF)-α and sTNF R II, according to the manufacturer's recommendations.
Assessment of neutrophil apoptosis
Apoptosis was assessed by flow cytometry following staining with FITC-annexin-V, as described previously [17].
Expression of cytoskeletal proteins
Neutrophils (1 × 106 cells/ml) harvested from buffer- or LPS-induced air pouch, were incubated in the presence or absence of agonists for the indicated periods of time, and then harvested for the preparation of cell lysates in Laemmli's sample buffer. Aliquots corresponding to 250 000 cells were loaded and run on 10% SDS-polyacrylamide gel electrophoresis (PAGE) and transferred from gel to polyvinyl difluoride (PVDF) membranes. Non-specific sites were blocked with 1% BSA in Tris-buffered saline (TBS)-Tween (25 mM Tris-HCl, pH 7·8, 190 mM NaCl, 0·15% Tween-20) 1 h at room temperature. Membranes were incubated with monoclonal anti-mouse cytoskeletal antibodies overnight at 4°C, followed by washes, and incubated with the appropriate horseradish peroxidase-labelled secondary antibody for 1 h at room temperature in fresh blocking solution as documented previously [12,17]. Membranes were washed three times with TBS-Tween, and bands were revealed with the enhanced chemiluminescence (ECL) Western blotting detection system (Amersham Pharmacia Biotech Inc. Montreal, Quebec, Canada).
Intracellular calcium mobilization
Changes in intracellular free calcium was assessed using the fluorescent probe Fluo-3-AM (Molecular Probes) as described previously [26]. Neutrophils (5 × 106 cells/ml), were incubated for 30 min at 37°C with 3 µM Fluo-3-AM in PBS-1% BSA. Cells were washed three times and suspended at 5 × 106 cells/ml in PBS-1% BSA. An aliquot of 50 µl was transferred to a FACS tube containing 450 µl prewarmed PBS-1% BSA + 1 mM CaCl2. Pre-warmed samples were loaded onto a FACScan flow cytometer, and fluorescence in FL1 was recorded for a period of ∼20 s. Then, 5 µl of 100X agonist was added to the tube and fluorescence was recorded during an additional 3 min. The calcium ionophore A23187 was used at a final concentration of 10 µM. PBS was used as negative control. A gate based on forward- and side-scatter was used to exclude debris.
Measurement of reactive oxygen species (ROS) production by DHR 123
Mitochondrial ROS production was assessed using dihydrorhodamine 123 (DHR123), as described previously [14,27]. Briefly, neutrophils were incubated at a final concentration of 1 × 106 cells/ml in HBSS-0·3% BSA containing 1 µM DHR123 for 5 min at 37°C before adding stimuli. Buffer or phorbol myristate acetate (PMA) (10−7 M) was then added and cells were incubated at 37°C for different intervals of time between 0 and 60 min. ROS production was analysed by FACScan by detecting fluorescence in the green channel at 530/30 nm (FL1) and 10 000 events per sample were analysed.
Phagocytosis
Phagocytosis of opsonized sheep red blood cells (SRBC) was performed essentially as described previously [25].
Statistics
Statistical analysis was performed with SigmaStat for Windows version 2·03 with a one-way analysis of variance (anova). Statistical significance was established at P < 0·05.
Results
Role of vimentin during an inflammatory response in vivo
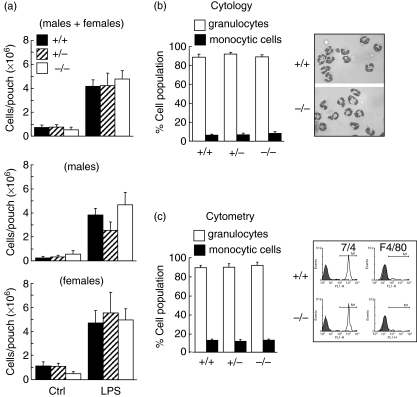
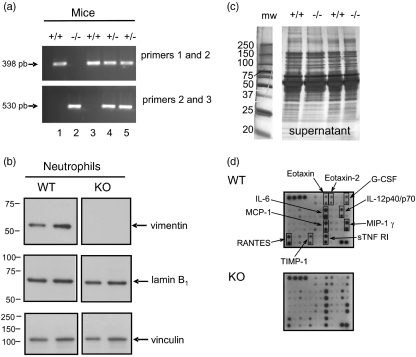
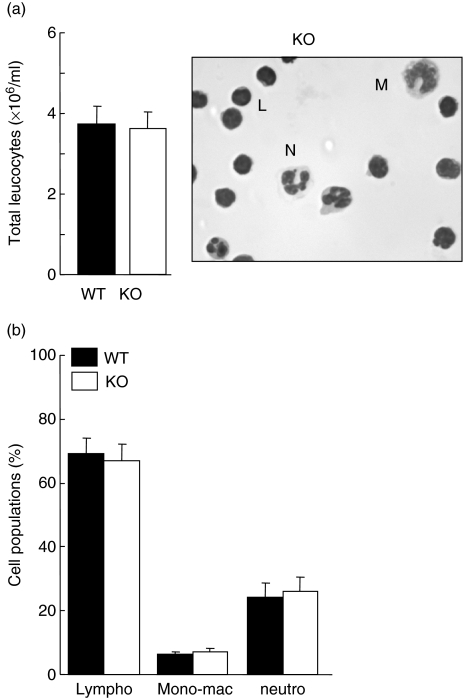
To investigate the role of vimentin during an inflammatory response, we used the murine air pouch model [18,25]. In this model, when LPS is administered into the pouch, more than ∼85% of the infiltrated cells are neutrophils, the rest being almost exclusively monocytic cells [25]. Thus, to investigate the role of vimentin during neutrophilic inflammation, we administered LPS into air pouches of wild-type (vim+/+), heterozygous (vim+/−) and homozygous (vim−/−) mice and harvested cells after 6 h of treatment. As expected, LPS significantly increased the number of leucocytes attracted into the air pouch of wild-type animals compared to control mice receiving the vehicle. Initially, experiments were performed randomly with males and females, depending upon the composition of the colony; results of the first panel represent the data obtained with both sexes (Fig. 1a, upper panel). Curiously, we obtained comparable results with all groups of mice, suggesting that vimentin is not involved in this response (Fig. 1). We then subdivided the results by analysing males and females separately, to determine whether or not there was a sexual dimorphism. As illustrated in the middle and bottom panels of Fig. 1a, no sexual dimorphism was observed. Cell populations were then identified by cytology (Hema-stain) (Fig. 1b) or flow cytometry (Fig. 1c). As illustrated in Fig. 1b, more than 85% of cells were neutrophils, and this was observed in vim−/−, vim+/− and vim−/− mice. Again, no sexual dimorphism was observed regarding the different cell populations (data not shown). Of note, both the nuclear and cellular morphology from vim−/− and vim+/+ neutrophils were identical (Fig. 1b, inset), indicating that absence of vimentin does not alter the typical polylobed neutrophil nucleus. As illustrated in Fig. 2, we confirmed the absence or presence of vimentin in randomly selected animals. Genotyping was performed systematically for all animals used in this study (Fig. 2a illustrates some examples). Although it has been reported originally that absence of vimentin was not compensated for by the expression of another IF protein [3], those experiments were performed using an antibody that reacts with most members of the IF proteins (except that lamin B was not mentioned), and these studies did not test neutrophils. Because the IF proteins expressed in neutrophils are only vimentin and lamin B, we examined the effects of absence of vimentin and whether or not this was compensated for by an increase in expression of lamin B in neutrophils. As illustrated in Fig. 2b, lamin B1 expression in neutrophils was identical in vim−/− and vim+/+ mice. Moreover, to support further the idea that there is no compensatory phenomenon at the protein level, we performed screening experiments to detect any major differences by comparing the profiles of intracellular protein expression. We found that they were identical in vim−/− and vim+/+ neutrophils (data not shown). By the same reasoning, we did not observe any differences in protein expression harvested from the air pouch exudates of knock-out or wild-type animals (Fig. 2c). To support further this latter observation, we performed an antibody array assay using a mouse inflammation antibody array map I and, again, we did not observe any differences in cytokine/chemokine expression in the exudates from vim−/−versus vim+/+ animals (Fig. 2d). Next, we verified if the basal number of total leucocytes, as well as lymphocytes, monocytic cells and neutrophils, differed between vim−/− and vim+/+ mice. As illustrated in Fig. 3, the total number of leucocytes was similar in both conditions, at approximately 4 × 106 cells/ml, as expected [24]. Of note, because we did not observe any difference between the number of leucocytes in males and females in vim−/−versus vim+/+ animals, the data are presented as a compilation of both males and females. More importantly, the number of neutrophils (representing ∼20% of total murine leucocytes), lymphocytes and monocytic cells were also similar in both conditions. This suggests that the identical neutrophilic inflammation observed in vim−/−versus vim+/+ mice was not the result of a compensatory phenomenon associated with an elevated basal elevated number of cells in knock-out animals.
Fig. 1.
Role of vimentin during an inflammatory response induced by lipopolysaccharide (LPS) in vivo. Dorsal air pouches were raised in vim+/+, vim+/− and vim−/− mice as described in Materials and methods before injecting 1 ml of the buffer (Ctrl) or LPS (1 µg/ml) directly into the pouch. Six hours later, the exudates were harvested and the number of emigrated total leucocytes was calculated (a) and separated into granulocytes (open bar) or monocytic cells (solid bar) (b, c). (a) Results are means ± standard error of the mean (s.e.m.) (n ≥ 14, males + females, five different experiments). (b) Cell identification was performed by cytology using the Hema-stain staining kit. Inset, a representative cytocentrifuged preparation of harvested cells illustrating that the majority of cells attracted by LPS are granulocytes in both vim−/− and vim+/+ mice. C, cells were stained with purified rat anti-mouse 7/4 monoclonal antibody directed against murine neutrophils (open bar) or rat anti-mouse F4/80 antigen antibody recognizing murine monocyte/macrophages (solid bar) and analysed by flow cytometry as described in Materials and methods. Inset, a representative data plotted in the bar graph (the appropriate isotypic controls are illustrated in grey). (b, c) Results are means ± s.e.m. (n = 6 animals, three different experiments). No significant differences were observed between vim+/+, vim+/− and vim−/− regarding the number of cells attracted as well as the percentages and subtype of cell populations (analysis of variance).
Fig. 2.
Evidence that the protein pattern expressions are identical in vim–/– and vim+/+ neutrophils. (a) Mice genotyping was performed systematically by polymerase chain reaction using the three different primers, as described in Materials and methods according to Eckes et al. [8]. The 1–2 primer pair allows amplification of a 398-base pairs (bp) fragment when the wild-type allele is present and the 2–3 primer pair allows amplification of a 530-bp fragment when the disrupted allele is present [8]. (b) Expression of the cytoskeletal proteins vimentin, lamin B1 and vinculin in harvested neutrophils following lipopolysaccharide (LPS)-induced murine air pouch. Results are representative of at least 23 animals (total). (c) Exudates were collected from the pouch and separated into two fractions: cells pellet (not shown) and supernatants. Proteins from were run by electrophoresis and gels were stained with silver staining for extracellular proteins (5–20% sodium dodecyl sulphate–polyacrylamide gel electrophoresis). Molecular weights are indicated on the left side. (b, c) Results are representative of at least 15 or 18 different animals, respectively. (d) Antibody array assay was performed with exudates collected and pooled from 13 LPS-induced air pouches for wild-type and knock-out mice (for a total of 4·6 ± 0·8 and 4·8 ± 0·9 leucocytes attracted/pouch, respectively). The cytokines/chemokines that are the most affected by LPS are boxed. Note the presence of two dots per analyte, as all cytokines/chemokines were tested in duplicate. No differences were noted when the intensity of each spot was compared with the corresponding positive control. The list of all cytokines/chemokines is detailed in the Materials and methods.
Fig. 3.
Mice lacking vimentin possess the same number of leucocytes as well as the same proportions of lymphocyte, monocytic and neutrophil cells. (a) Blood collection was performed by cardiac puncture from vim+/+ (wild-type) or vim−/− (knock-out) animals and leucocyte counts were assessed by cytology as described in Materials and methods. (b) Differential cell count was performed with an aliquot of 0·5 × 106 leucocytes cytocentrifuged on microscope slides and coloured with the Hema-3 stain set in order to differentiate lymphocytes (Lympho), monocytes-macrophages (Mono-mac) and neutrophils (neutro). Results are means ± standard error of the mean (n = 10 mice/group, three separate experiments). Inset, note the preponderance of murine lymphocytes (L) in the blood as opposed to neutrophils (N) or monocytes–macrophage (M), as expected [24].
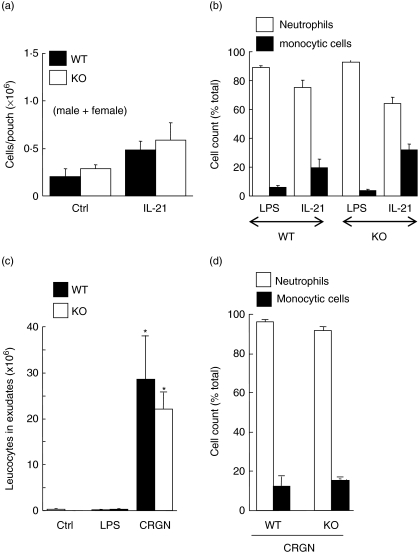
Because we found that LPS induced a similar neutrophilic inflammation in both vim−/− and vim+/+ mice, we decided to test whether or not the absence of vimentin alters the attraction of mononuclear cells in response to IL-21. As expected, IL-21 attracted both neutrophils and monocytic cells in wild-type animals, compared to LPS which attracted almost exclusively neutrophils (Fig. 4a,b). However, the response of IL-21 appeared to be less potent in these mice when compared to the CD−1 mice used previously [25]. Nevertheless, no significant difference was observed when comparing the leucocyte infiltration induced by IL-21 in vim−/− and vim+/+, indicating that our previous results obtained (Fig. 1) were not restricted to LPS activation or neutrophil cell attraction. During the experiments with IL-21, LPS was used in parallel and the number of cells attracted (consisting almost exclusively of neutrophils) was always greater than 4 × 106 cells/pouch (data not shown). It should be noted that, although results were obtained from both sexes, we verified the results separately and did not observe any sexual dimorphism. This is also true for all the next parameters investigated in the present study. Consequently, for simplicity, all the data for the rest of the manuscript will not be separated into males or females. As both the LPS- and IL-21-induced neutrophilic infiltration in the murine air pouch model is normally resolved after 24 h by a mechanism that remains obscure (neutrophil counts dramatically diminished after 9–12 h and were almost undetectable after 24 h) [25], we verified whether or not neutrophils devoid of vimentin would respond normally to carrageenan (CRGN), an agent that is not only more powerful than LPS but that increased the persistence of neutrophils into pouches for up to 24 h. The leucocyte inflammation after 24 h was similar in both vim−/− and vim+/+ mice because CRGN attracted more than ∼20 × 106 cells/pouch (Fig. 4c,d). It is important to mention that almost no cells are detected in the pouch after 24 h of LPS treatment in both knock-out and wild-type animals, indicating that the mechanisms for the resolution of inflammation are also not altered in the absence of vimentin.
Fig. 4.
Absence of vimentin does not alter neutrophil and monocytic cell influx in vivo. (a, b) Dorsal air pouches were raised in vim+/+ and vim−/− mice before injecting 1 ml of the buffer (Ctrl), lipopolysaccharide (LPS) (1 µg/ml) or interleukin (IL)-21 (100 ng/ml) directly into the pouch. Six hours later, the exudates were harvested and the number of emigrated total leucocytes was calculated (a). Results are means ± standard error of the mean (s.e.m.) (n = 8, two separate experiments) and include males and females as no sexual dimorphism was observed. Differential counts were assessed by cytology (b, n ≥ 4 mice/group, pool of two experiments). (c, d) Dorsal air pouches were raised in vim+/+ (wild-type) and vim−/− (knock-out) mice before injecting 1 ml of the buffer (Ctrl), LPS (1 µg/ml) or carrageenan (CRGN, 1% in Hank's balanced salt solution) directly into the air pouch. Twenty-four hours later, the exudates were harvested and the number of emigrated total leucocytes was calculated (c). Results are means ± s.e.m. (n = 3 mice/group, two separate experiments) and include males and females. Differential counts were assessed by cytology (d). No significant differences were observed between wild-type and knock-out mice regarding the number of cells attracted as well as the percentages and subtype of cell populations (analysis of variance).
Role of vimentin in neutrophil apoptosis
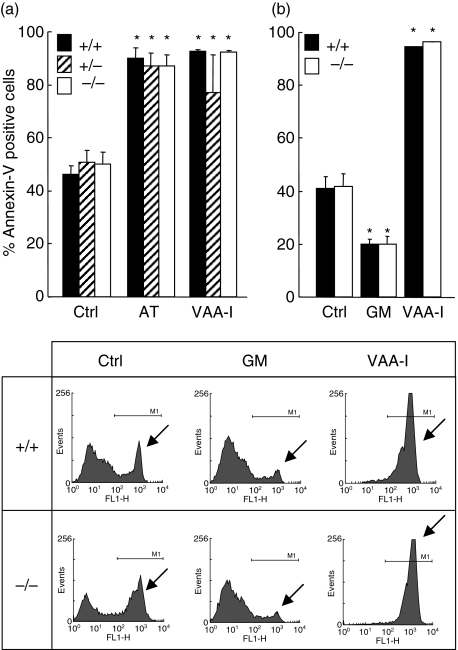
Cells were harvested from LPS-induced air pouches from vim−/−, vim+/− and vim+/+ mice and incubated in vitro with different agonists. Surprisingly, the rate of apoptosis was similar during spontaneous, V. album agglutinin-I (VAA-I-) or arsenic trioxide (AT)-induced apoptosis in vim−/−, vim+/− and vim+/+ neutrophils, indicating that this protein does not play a major role in the induction of apoptosis (Fig. 5a). As illustrated in Fig. 5b, the potent anti-apoptotic cytokine GM-CSF [28] was found to delay neutrophil apoptosis similarly in vim−/− and vim+/+ neutrophils, indicating that vimentin is not required for the regulation of neutrophil apoptosis.
Fig. 5.
The spontaneous apoptotic rate and its modulation by pro- or anti-apoptotic agents are not altered in neutrophils devoid of vimentin ex vivo. Neutrophils from vim+/+, vim+/− and vim−/− mice were harvested after lipopolysaccharide (LPS)-induced air pouch and incubated in vitro for 22 h in the presence of buffer (Ctrl), the pro-apoptotic agents arsenic trioxide (AT, 5 µM) or Viscum album agglutinin-I (VAA-I, 1000 ng/ml) or the anti-apoptotic cytokine granulocyte–macrophage colony-stimulating factor (GM-CSF) (GM, 65 ng/ml) and apoptosis was assessed by flow cytometry by evaluating the number of fluorescein isothiocyanate (FITC)-annexin-V positive cells. (a) Results are means ± standard error of the mean (n = 10 mice/group, four separate experiments) and (b) (n = 5 mice/group, two separate experiments). Inset, representative experiment plotted in the bar graph. Arrows, illustrates the apoptotic cell population. *P < 0·05 versus control (Ctrl) by analysis of variance. No significant differences were observed between wild-type and knock-out mice.
Role of vimentin in intracellular calcium mobilization
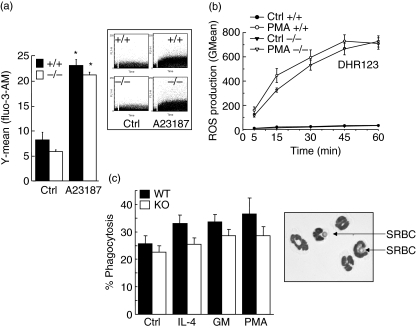
Next, we decided to investigate the possibility that vimentin exerts a role in neutrophil cell physiology during a rapid response, as the above responses (neutrophilic inflammation and apoptosis) require several hours. Calcium influx, a very rapid response occurring in neutrophils, is activated for few seconds to several minutes by a variety of agonists [26]. As illustrated in Fig. 6a, calcium influx was increased significantly following treatment with ionophore A23187. However, this increase was similar in both vim−/− and vim+/+ neutrophils, indicating that absence of vimentin did not interfere with this rapid response.
Fig. 6.
Role of vimentin in intracellular calcium mobilization, reactive oxygen species (ROS) generation and phagocytosis. Neutrophils from vim+/+ (wild-type) and vim−/− (knock-out) mice were harvested from lipopolysaccharide (LPS)-induced air pouches and adjusted at a concentration of 5 × 106 cells/ml, before being incubated for calcium measurements as detailed in Materials and methods. The calcium ionophore A23187 was used at a final concentration of 10 µM. Phosphate-buffered saline (PBS) was used as a negative control. Inset, representative data plotted in the bar graph. Results are means ± standard error of the mean (s.e.m.) (n = 7, total). (b) Harvested cells were incubated in vitro in the presence or absence (Ctrl) of phorbol 12-myristate 13-acetate (PMA) (10−7 M) for the indicated periods of time. Mitochondrial ROS production was assessed by flow cytometry using DHR123. Results are means ± s.e.m. (n = 13 mice/group, three separate experiments). (c) Neutrophils from vim+/+ (wild-type) and vim−/− (knock-out) mice were harvested from LPS-induced air pouches and incubated in vitro in the presence of buffer (Ctrl), interleukin (IL)-4 (100 ng/ml), granulocyte–macrophage colony-stimulating factor (GM-CSF) (65 ng/ml, GM) or PMA (30 ng/ml) and phagocytosis was assessed by by counting the number of cells ingesting at least one opsonized-sheep red blood cell (SRBC). Results are means ± s.e.m. (n = 3 mice/group, four separate experiments) and are expressed as percentage of phagocytosis. Inset, vim−/− neutrophils ingesting opsonized SRBC (arrows). *P < 0·05 versus control (Ctrl) by analysis of variance. No significant differences were observed between wild-type and knock-out neutrophils.
Absence of vimentin does not alter ROS production
Next, we investigated the role of vimentin in another rapid neutrophil function: the respiratory burst. Mitochondrial ROS production was assessed by flow cytometry using DHR123 [14,27]. PMA was used as a positive control [14,27]. As illustrated in Fig. 6b, the PMA-induced intracellular ROS did not differ significantly in vimentin-deficient neutrophils when compared to wild-type neutrophils after 15, 30, 45 and 60 min of stimulation.
Role of vimentin in phagocytosis
To elucidate the role of vimentin in phagocytosis, we evaluated the capacity of neutrophils to ingest opsonized-SRBC [25]. We have studied the basal ability of vim+/+ and vim−/− neutrophils to exert phagocytosis as well as in IL-4-, GM-CSF- or PMA-induced cells. The results indicate that the levels of phagocytosis in neutrophils devoid of vimentin were slightly, but non-significantly, decreased, whether or not the cells were activated with IL-4, GM-CSF or PMA (Fig. 6c).
Discussion
Prior to initiation of this study we expected to find major differences between an inflammatory response in vivo, including an increased potency of different proinflammatory agents in vim−/− mice caused by a refractory apoptotic mechanism increasing the neutrophilic inflammation in vivo. This was expected not only because of the importance of vimentin in the cell architecture and a variety of cell functions and responses, but also because we have demonstrated recently that vimentin is an important target cleaved by caspases in spontaneous or agent-induced neutrophil apoptosis [12,14–18]. In addition, some vimentin-cleaved fragments have been found to be pro-apoptotic themselves [29]. Moreover, we reported recently that vimentin is expressed on the cell surface of apoptotic neutrophils [17], suggesting that it may participate in the recognition process of apoptotic neutrophils by phagocytes for the resolution of inflammation or in the elaboration of autoantibodies against vimentin sequestered normally inside the cell. In addition, mice deficient in gelsolin (Gsn−/−), another cytoskeletal protein cleaved by caspases in apoptotic neutrophils [17,30], were found to possess twice the number of neutrophils due to the fact that they were refractory for apoptosis.
In the murine air pouch model, all steps required for the migration of neutrophils from circulation to reach an inflammatory site (the pouch) are involved, including rolling, adhesion, tight binding, diapedesis and migration [31–33]. Because we report here that neutrophils devoid of vimentin are attracted at the same magnitude as normal vim+/+ neutrophil cells in response to LPS, it is highly plausible that if any neutrophil functions/responses are altered due to a lack of vimentin, this does not compromise an acute inflammatory response in vivo. When IL-21 was used in the murine air pouch model, we also found an identical response in vim+/+ and vim−/− mice, indicating that the same observation made for neutrophils is also true for the monocytic cell population. LPS is known to mediate its effects via Toll-like receptor-4 (TLR-4), while IL-21 binds to its receptor composed of IL-21Rα and CD132, a subunit common to receptors for IL-2, IL-7, IL-9 and IL-15. Thus, these two proinflammatory agents act by different mechanisms. In contrast to our results, a slightly increased proportion of incoming neutrophils in vim−/− mice, when compared with resident macrophages, has been reported in a mild peritonitis murine model (supplementary information, figure S2c in [9]). However, in contrast to the severe LPS-induced air pouch model that we have used in which neutrophil influx occurred after few hours, the cells used in the peritonitis model to evaluate the inflammatory state were harvested after 25 h. At this period of time, the leucocyte influx is no longer observable in most of the agent-induced air pouch, including LPS [18] and IL-21 [25]. This indicates that, unlike neutrophils (this report), macrophage functions can be altered in vim−/− mice when compared with vim+/+ macrophages, because in this model resident macrophages may be the primary target of an inflammatory agent. Interestingly, it has been reported that vimentin is secreted by activated human macrophages and that this protein is involved in immune functions of this cell type, as extracellular vimentin was found to be involved in bacterial killing and the generation of oxidative metabolites [23].
Because the response to CRGN was identical in vim−/−versus vim+/+ animals, we concluded that vim−/− neutrophils and vim−/− monocytes–macrophages (in the case of IL-21) are attracted and leave the air pouch at the same rate as vim+/+ neutrophils. Recently, using vim−/− mice, Nieminen et al. [9] found a role for vimentin in lymphocyte adhesion and transcellular migration. Interestingly, utilizing intravital microscopy with the cremaster muscle, in which more than 90% of interacting leucocytes in this model are neutrophils, they found no difference in the rolling flux, but the velocity of the rolling cells was almost three times higher in vim−/− mice than in littermate controls [9]. Also, the number of adherent cells was significantly lower in vim−/− mice but the number of transmigrating cells was higher. Although we did not conduct such experiments in the present study, we cannot deny the possibility that a similar mechanism operates in our model that would compensate and result in identical acute inflammatory responses in vivo observed in both vim−/− and vim+/+ animals. However, because the observed inflammatory response was identical in knock-out versus wild-type animals in response to three different agents attracting different cell types (LPS, IL-21) and at different periods of time (CRGN), it is difficult to imagine that such a phenomenon alone could explained the absence of difference in vim−/− and vim+/+ mice, regarding the complex process of leucocyte inflammation in vivo involving various parameters. Our results indicate that a potential compensatory phenomenon occurring in vim−/− mice could not be explained by an increased number of leucocytes (particularly neutrophils) in knock-out animals or by an exaggerated response of these cells. As mentioned above, several steps are required for leucocytes to reach the air pouch. In addition, cells have to leave and/or to be eliminated from the pouch by a mechanism that is not understood fully, but which probably involves neutrophil apoptosis and clearance of apoptotic cells by professional phagocytes [9]. The results of the present study demonstrated clearly that the ability of vim−/− neutrophils to undergo apoptosis was not altered in comparison with vim+/+ cells. This is true for spontaneous apoptosis as well as agent-induced apoptosis (VAA-I and AT). In a recent study, mutations in vimentin were found to disrupt the cytoskeleton in fibroblasts and delay execution of apoptosis [34]. This allows the possibility that expression of a mutated form of vimentin may alter apoptosis. Although vimentin autoantibodies are associated with different rheumatic diseases, there is no human disease, to date, associated with a vimentin mutation.
IF proteins are known to be involved in the architecture of the nucleus. In particular, in granulocytes, the characteristic polylobed nucleus is thought to be due to the loss of lamin A/C expression, but not lamin B, during the differentiation of promyelocytes (ovoid nucleus) towards neutrophils (lobulated nucleus). This is supported further in vivo in patients suffering from Pelger–Huët anomaly, characterized by neutrophils with a nuclear hyposegmentation, where the lamin A/C-cleaving activity of caspase-6 is decreased markedly in comparison with neutrophils from healthy individuals [35]. Recently, mutations in the gene encoding the lamin B receptor were associated with the Pelger–Huët anomaly [36]. In the present study, the cell shape, as well as the appearance and morphology of the nucleus were not different in knock-out versus wild-type neutrophils. In contrast, a single study has reported that the vimentin filament system affects the shape of the nucleus in human epithelial-like SW-13 cells [37]. This conclusion emanates from electron microscopy experiments in which the nuclear morphology in vimentin-negative cells was characterized by large fold invaginations in comparison with vimentin-positive cells, possessing a more regular or smooth nuclear morphology. This is an example reporting a difference between vim−/− and vim+/+ cells, but because no correlations were made with an in vivo defect, these results are difficult to interpret in the context of a complex biological process such as an inflammatory response in vivo. We have verified if the ability of vim−/− neutrophils to migrate into the air pouch in response to LPS was compensated for by the local production of proinflammatory molecules released in the exudates. The profiles of protein expression were identical in wild-type versus knock-out animals. However, this does not rule out the possibility that one or a few chemokines are over-expressed in vim−/− mice, but the mouse inflammation antibody array membrane we used covered major chemokines thought to be involved in LPS-induced air pouch.
There is little information in the literature regarding the use of knock-out mice to study the role of a given cytoskeletal protein in neutrophil phagocytosis. However, Gsn– mice have been used to demonstrate clearly selective inhibition of IgG-mediated phagocytosis in gelsolin-deficient murine neutrophils [38]. In this latter study, the phagocytosis of complement opsonized yeast was affected only minimally, but phagocytosis of IgG-opsonized yeast was markedly reduced, indicating the importance of this microfilament-associated protein in Fc-mediated phagocytosis. More recently, Gsn– mice were used to show that gelsolin mediates collagen phagocytosis through a rac-dependent mechanism [39,40]. In this study, using vim−/− mice, we have demonstrated for the first time that vimentin is not involved in Fc-mediated neutrophil phagocytosis.
Taken together, the results of the present study argue against a simple compensatory mechanism proposed frequently to explain the absence of a major biological effect (ultimately the death) in an animal devoid of a given protein. We believe that this study represents a good example of the notion that if a compensatory mechanism exists in vim−/− mice, at least for an acute inflammatory response, it should be complex and multi-parametric. Here we provide the following parameters which must be excluded but which support our point of view: (i) vim−/− mice possessed the same number of leucocytes and neutrophils as vim+/+ mice; (ii) vim−/− neutrophils expressed the same level of lamin B1 protein as vim+/+ mice; (iii) the cell morphology and the nucleus shape were identical in vim−/−versus vim+/+ mice; (iv) vim−/− neutrophils isolated from air-pouches possessed the same electrophoretic pattern of protein expression (intracellular) and the profiles of proteins detected in exudates were identical in knock-out and wild-type animals; (v) the inflammatory response was identical in vim−/−versus vim+/+ animals in terms of cell numbers and populations attracted in response to the three proinflammatory molecules, LPS, IL-21 and CRGN; (vi) the same apoptotic rates (spontaneous, induced or delayed) were observed in neutrophils whether they were isolated from knock-out or wild-type animals; (vii) there was no alteration of neutrophil functions including calcium mobilization, total ROS generation and phagocytosis; and (viii) there was no sexual dimorphism.
Acknowledgments
This study was partly supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) and Fonds de la Recherche en Santé du Québec (FRSQ). E. M. was supported by a PhD, FRSQ and Fondation Armand-Frappier studentship awards. D. G. is a Scholar from FRSQ. We thank Mary Gregory for reading this manuscript.
References
- 1.Bruel A, Paschke S, Jainta S, et al. Remodeling of vimentin cytoskeleton correlates with enhanced motility of promyelocytic leukemia cells during differentiation induced by retinoic acid. Anticancer Res. 2001;21:3973–80. [PubMed] [Google Scholar]
- 2.Vergnes L, Peterfy M, Bergo MO, Young SG, Reue K. Lamin B1 is required for mouse development and nuclear integrity. Proc Natl Acad Sci USA. 2004;101:10428–33. doi: 10.1073/pnas.0401424101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colucci-Guyon E, Portier MM, Dunia I, Paulin D, Pournin S, Babinet C. Mice lacking vimentin develop and reproduce without an obvious phenotype. Cell. 1994;79:679–94. doi: 10.1016/0092-8674(94)90553-3. [DOI] [PubMed] [Google Scholar]
- 4.Terzi F, Henrion D, Colucci-Guyon E, et al. Reduction of renal mass is lethal in mice lacking vimentin. Role of endothelin–nitric oxide imbalance. J Clin Invest. 1997;100:1520–8. doi: 10.1172/JCI119675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schiffers PM, Henrion D, Boulanger CM, et al. Altered flow-induced arterial remodeling in vimentin-deficient mice. Arterioscler Thromb Vasc Biol. 2000;20:611–6. doi: 10.1161/01.atv.20.3.611. [DOI] [PubMed] [Google Scholar]
- 6.Eckes B, Colucci-Guyon E, Smola H, et al. Impaired wound healing in embryonic and adult mice lacking vimentin. J Cell Sci. 2000;113:2455–62. doi: 10.1242/jcs.113.13.2455. [DOI] [PubMed] [Google Scholar]
- 7.Langa F, Kress C, Colucci-Guyon E, et al. Teratocarcinomas induced by embryonic stem (ES) cells lacking vimentin: an approach to study the role of vimentin in tumorigenesis. J Cell Sci. 2000;113:3463–72. doi: 10.1242/jcs.113.19.3463. [DOI] [PubMed] [Google Scholar]
- 8.Eckes B, Dogic D, Colucci-Guyon E, et al. Impaired mechanical stability, migration and contractile capacity in vimentin-deficient fibroblasts. J Cell Sci. 1998;111:1897–907. doi: 10.1242/jcs.111.13.1897. [DOI] [PubMed] [Google Scholar]
- 9.Nieminen M, Henttinen T, Merinen M, Marttila-Ichihara F, Eriksson JE, Jalkanen S. Vimentin function in lymphocyte adhesion and transcellular migration. Nat Cell Biol. 2006;8:156–62. doi: 10.1038/ncb1355. [DOI] [PubMed] [Google Scholar]
- 10.Lavastre V, Girard D. Tributyltin induces human neutrophil apoptosis and selective degradation of cytoskeletal proteins by caspases. J Toxicol Environ Health A. 2002;65:1013–24. doi: 10.1080/00984100290071270. [DOI] [PubMed] [Google Scholar]
- 11.Lavastre V, Roberge CJ, Pelletier M, Gauthier M, Girard D. Toxaphene, but not beryllium, induces human neutrophil chemotaxis and apoptosis via reactive oxygen species (ROS): involvement of caspases and ROS in the degradation of cytoskeletal proteins. Clin Immunol. 2002;104:40–8. doi: 10.1006/clim.2002.5226. [DOI] [PubMed] [Google Scholar]
- 12.Lavastre V, Pelletier M, Saller R, Hostanska K, Girard D. Mechanisms involved in spontaneous and Viscum album agglutinin-I-induced human neutrophil apoptosis: Viscum album agglutinin-I accelerates the loss of antiapoptotic Mcl-1 expression and the degradation of cytoskeletal paxillin and vimentin proteins via caspases. J Immunol. 2002;168:1419–27. doi: 10.4049/jimmunol.168.3.1419. [DOI] [PubMed] [Google Scholar]
- 13.Savoie A, Lavastre V, Pelletier M, Hajto T, Hostanska K, Girard D. Activation of human neutrophils by the plant lectin Viscum album agglutinin-I: modulation of de novo protein synthesis and evidence that caspases are involved in induction of apoptosis. J Leukoc Biol. 2000;68:845–53. [PubMed] [Google Scholar]
- 14.Binet F, Cavalli H, Moisan E, Girard D. Arsenic trioxide (AT) is a novel human neutrophil pro-apoptotic agent: effects of catalase on AT-induced apoptosis, degradation of cytoskeletal proteins and de novo protein synthesis. Br J Haematol. 2006;132:349–58. doi: 10.1111/j.1365-2141.2005.05866.x. [DOI] [PubMed] [Google Scholar]
- 15.Lavastre V, Chiasson S, Cavalli H, Girard D. Viscum album agglutinin-I (VAA-I) induces apoptosis and degradation of cytoskeletal proteins in human leukemia PLB-985 and X-CGD cells via caspases: lamin B1 is a novel target of VAA-I. Leuk Res. 2005;29:1443–53. doi: 10.1016/j.leukres.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 16.Lavastre V, Chiasson S, Cavalli H, Girard D. Viscum album agglutinin-I induces apoptosis and degradation of cytoskeletal proteins via caspases in human leukaemia eosinophil AML14.3D10 cells: differences with purified human eosinophils. Br J Haematol. 2005;130:527–35. doi: 10.1111/j.1365-2141.2005.05633.x. [DOI] [PubMed] [Google Scholar]
- 17.Moisan E, Girard D. Cell surface expression of intermediate filament proteins vimentin and lamin B1 in human neutrophil spontaneous apoptosis. J Leukoc Biol. 2006;79:489–98. doi: 10.1189/jlb.0405190. [DOI] [PubMed] [Google Scholar]
- 18.Lavastre V, Cavalli H, Ratthe C, Girard D. Anti-inflammatory effect of Viscum album agglutinin-I (VAA-I): induction of apoptosis in activated neutrophils and inhibition of lipopolysaccharide-induced neutrophilic inflammation in vivo. Clin Exp Immunol. 2004;137:272–8. doi: 10.1111/j.1365-2249.2004.02545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansson GK, Lagerstedt E, Bengtsson A, Heideman M. IgG binding to cytoskeletal intermediate filaments activates the complement cascade. Exp Cell Res. 1987;170:338–50. doi: 10.1016/0014-4827(87)90311-9. [DOI] [PubMed] [Google Scholar]
- 20.Hansson GK, Starkebaum GA, Benditt EP, Schwartz SM. Fc-mediated binding of IgG to vimentin-type intermediate filaments in vascular endothelial cells. Proc Natl Acad Sci USA. 1984;81:3103–7. doi: 10.1073/pnas.81.10.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albrecht DL, Mills JW, Noelle RJ. Membrane Ig–cytoskeletal interactions. III. Receptor cross-linking results in the formation of extensive filamentous arrays of vimentin. J Immunol. 1990;144:3251–6. [PubMed] [Google Scholar]
- 22.Brown MJ, Hallam JA, Colucci-Guyon E, Shaw S. Rigidity of circulating lymphocytes is primarily conferred by vimentin intermediate filaments. J Immunol. 2001;166:6640–6. doi: 10.4049/jimmunol.166.11.6640. [DOI] [PubMed] [Google Scholar]
- 23.Mor-Vaknin N, Punturieri A, Sitwala K, Markovitz DM. Vimentin is secreted by activated macrophages. Nat Cell Biol. 2003;5:59–63. doi: 10.1038/ncb898. [DOI] [PubMed] [Google Scholar]
- 24.Doeing DC, Borowicz JL, Crockett ET. Gender dimorphism in differential peripheral blood leukocyte counts in mice using cardiac, tail, foot, and saphenous vein puncture methods. BMC Clin Pathol. 2003;3:3. doi: 10.1186/1472-6890-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pelletier M, Bouchard A, Girard D. In vivo and in vitro roles of IL-21 in inflammation. J Immunol. 2004;173:7521–30. doi: 10.4049/jimmunol.173.12.7521. [DOI] [PubMed] [Google Scholar]
- 26.Merritt JE, McCarthy SA, Davies MP, Moores KE. Use of fluo-3 to measure cytosolic Ca2+ in platelets and neutrophils. Loading cells with the dye, calibration of traces, measurements in the presence of plasma, and buffering of cytosolic Ca2+ Biochem J. 1990;269:513–9. doi: 10.1042/bj2690513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Falcone FH, Rossi AG, Sharkey R, Brown AP, Pritchard DI, Maizels RM. Ascaris suum-derived products induce human neutrophil activation via a G protein-coupled receptor that interacts with the interleukin-8 receptor pathway. Infect Immun. 2001;69:4007–18. doi: 10.1128/IAI.69.6.4007-4018.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yasui K, Sekiguchi Y, Ichikawa M, et al. Granulocyte macrophage colony-stimulating factor delays neutrophil apoptosis and primes its function through Ia-type phosphoinositide 3-kinase. J Leukoc Biol. 2002;72:1020–2. [PubMed] [Google Scholar]
- 29.Byun Y, Chen F, Chang R, Trivedi M, Green KJ, Cryns VL. Caspase cleavage of vimentin disrupts intermediate filaments and promotes apoptosis. Cell Death Differ. 2001;8:443–50. doi: 10.1038/sj.cdd.4400840. [DOI] [PubMed] [Google Scholar]
- 30.Kothakota S, Azuma T, Reinhard C, et al. Caspase-3-generated fragment of gelsolin: effector of morphological change in apoptosis. Science. 1997;278:294–8. doi: 10.1126/science.278.5336.294. [DOI] [PubMed] [Google Scholar]
- 31.Merinen M, Irjala H, Salmi M, Jaakkola I, Hanninen A, Jalkanen S. Vascular adhesion protein-1 is involved in both acute and chronic inflammation in the mouse. Am J Pathol. 2005;166:793–800. doi: 10.1016/S0002-9440(10)62300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Werr J, Johansson J, Eriksson EE, Hedqvist P, Ruoslahti E, Lindbom L. Integrin alpha(2) beta(1) (VLA-2) is a principal receptor used by neutrophils for locomotion in extravascular tissue. Blood. 2000;95:1804–9. [PubMed] [Google Scholar]
- 33.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2:612–9. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 34.Schietke R, Brohl D, Wedig T, Mucke N, Herrmann H, Magin TM. Mutations in vimentin disrupt the cytoskeleton in fibroblasts and delay execution of apoptosis. Eur J Cell Biol. 2006;85:1–10. doi: 10.1016/j.ejcb.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 35.Yabuki M, Miyake T, Doi Y, et al. Role of nuclear lamins in nuclear segmentation of human neutrophils. Physiol Chem Phys Med NMR. 1999;31:77–84. [PubMed] [Google Scholar]
- 36.Hoffmann K, Dreger CK, Olins AL, et al. Mutations in the gene encoding the lamin B receptor produce an altered nuclear morphology in granulocytes (Pelger–Huet anomaly) Nat Genet. 2002;31:410–4. doi: 10.1038/ng925. [DOI] [PubMed] [Google Scholar]
- 37.Sarria AJ, Lieber JG, Nordeen SK, Evans RM. The presence or absence of a vimentin-type intermediate filament network affects the shape of the nucleus in human SW-13 cells. J Cell Sci. 1994;107(6):1593–607. doi: 10.1242/jcs.107.6.1593. [DOI] [PubMed] [Google Scholar]
- 38.Serrander L, Skarman P, Rasmussen B, et al. Selective inhibition of IgG-mediated phagocytosis in gelsolin-deficient murine neutrophils. J Immunol. 2000;165:2451–7. doi: 10.4049/jimmunol.165.5.2451. [DOI] [PubMed] [Google Scholar]
- 39.Arora PD, Chan MW, Anderson RA, Janmey PA, McCulloch CA. Separate functions of gelsolin mediate sequential steps of collagen phagocytosis. Mol Biol Cell. 2005;16:5175–90. doi: 10.1091/mbc.E05-07-0648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arora PD, Glogauer M, Kapus A, Kwiatkowski DJ, McCulloch CA. Gelsolin mediates collagen phagocytosis through a rac-dependent step. Mol Biol Cell. 2004;15:588–99. doi: 10.1091/mbc.E03-07-0468. [DOI] [PMC free article] [PubMed] [Google Scholar]