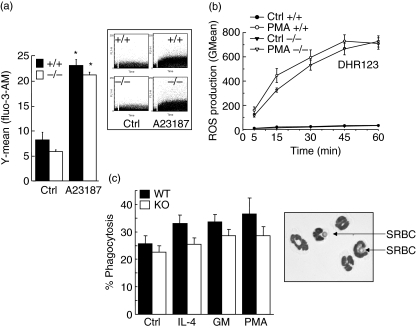
Fig. 6.
Role of vimentin in intracellular calcium mobilization, reactive oxygen species (ROS) generation and phagocytosis. Neutrophils from vim+/+ (wild-type) and vim−/− (knock-out) mice were harvested from lipopolysaccharide (LPS)-induced air pouches and adjusted at a concentration of 5 × 106 cells/ml, before being incubated for calcium measurements as detailed in Materials and methods. The calcium ionophore A23187 was used at a final concentration of 10 µM. Phosphate-buffered saline (PBS) was used as a negative control. Inset, representative data plotted in the bar graph. Results are means ± standard error of the mean (s.e.m.) (n = 7, total). (b) Harvested cells were incubated in vitro in the presence or absence (Ctrl) of phorbol 12-myristate 13-acetate (PMA) (10−7 M) for the indicated periods of time. Mitochondrial ROS production was assessed by flow cytometry using DHR123. Results are means ± s.e.m. (n = 13 mice/group, three separate experiments). (c) Neutrophils from vim+/+ (wild-type) and vim−/− (knock-out) mice were harvested from LPS-induced air pouches and incubated in vitro in the presence of buffer (Ctrl), interleukin (IL)-4 (100 ng/ml), granulocyte–macrophage colony-stimulating factor (GM-CSF) (65 ng/ml, GM) or PMA (30 ng/ml) and phagocytosis was assessed by by counting the number of cells ingesting at least one opsonized-sheep red blood cell (SRBC). Results are means ± s.e.m. (n = 3 mice/group, four separate experiments) and are expressed as percentage of phagocytosis. Inset, vim−/− neutrophils ingesting opsonized SRBC (arrows). *P < 0·05 versus control (Ctrl) by analysis of variance. No significant differences were observed between wild-type and knock-out neutrophils.